Bechgaard salt
Encyclopedia
A Bechgaard salt is any one of a number of organic charge-transfer complexes that exhibit superconductivity
at low temperatures . They are named for chemist Klaus Bechgaard
, who was one of the first scientists to synthesize them and demonstrate their superconductivity with the help of physicist Denis Jérôme . Most Bechgaard salt superconductors are extremely low temperature, and lose superconductivity above the 1-2 K range, although the most successful compound in this class superconducts up to almost 12 K.
All Bechgaard salts are formed using a small, planar organic molecule as an electron donor
, with any of a number of electron acceptor
s (like perchlorate (ClO4) or tetracyanoethylene
(TCNE)). All the organic electron donors contain multiply conjugated
heterocycles with a number of properties, including planarity, low ionization potential
and good orbital overlap between heteroatoms in neighboring donor molecules. These properties help the final salt conduct electrons by shuttling them through the orbital vacancies left in the donor molecules.
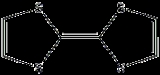
All Bechgaard salts have a variation on a single tetrathiafulvalene
motif - different superconductors have been made with appendages to the motif, or using a tetraselenafulvalene
center instead (which is a related compound), but all bear this general structural similarity.
There are a wide range of other organic superconductor
s including many other charge-transfer complexes.
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
at low temperatures . They are named for chemist Klaus Bechgaard
Klaus Bechgaard
Klaus Bechgaard is a Danish scientist and chemist, noted for being one of the first scientists in the world to synthesize a number of organic charge transfer complexes and demonstrate their superconductivity, threreof the name Bechgaard salt...
, who was one of the first scientists to synthesize them and demonstrate their superconductivity with the help of physicist Denis Jérôme . Most Bechgaard salt superconductors are extremely low temperature, and lose superconductivity above the 1-2 K range, although the most successful compound in this class superconducts up to almost 12 K.
All Bechgaard salts are formed using a small, planar organic molecule as an electron donor
Electron donor
An electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process....
, with any of a number of electron acceptor
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
s (like perchlorate (ClO4) or tetracyanoethylene
Tetracyanoethylene
Tetracyanoethylene is a clear colored organic compound consisting of ethylene with the four hydrogen atom replaced with cyano groups. It is an important member of the cyanocarbons.-Synthesis and reactions:...
(TCNE)). All the organic electron donors contain multiply conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
heterocycles with a number of properties, including planarity, low ionization potential
Ionization potential
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
and good orbital overlap between heteroatoms in neighboring donor molecules. These properties help the final salt conduct electrons by shuttling them through the orbital vacancies left in the donor molecules.
All Bechgaard salts have a variation on a single tetrathiafulvalene
Tetrathiafulvalene
Tetrathiafulvalene is a organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, 2, by replacement of four CH groups with sulfur atoms...
motif - different superconductors have been made with appendages to the motif, or using a tetraselenafulvalene
Fulvalene
A fulvalene is a hydrocarbon obtained by formally cross-conjugating two rings through a common exocyclic double bond. The name is derived from the similarly structured fulvenes which lack one ring...
center instead (which is a related compound), but all bear this general structural similarity.
There are a wide range of other organic superconductor
Organic superconductor
In physical chemistry and condensed matter physics, an organic superconductor is an organic compound which exhibits superconductivity at low temperatures...
s including many other charge-transfer complexes.

