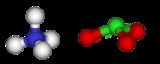
Ammonium chlorate
Encyclopedia
Ammonium chlorate is an inorganic compound
with the formula NH4ClO3.
It is obtained by neutralizing chloric acid
with either ammonia
or ammonium carbonate
, or by precipitating barium
, strontium
or calcium
chlorates with ammonium carbonate or ammonium sulfate, producing the respective carbonate or sulfate precipitate and an ammonium chlorate solution. Ammonium chlorate crystallizes in small needles, readily soluble in water.
On heating, ammonium chlorate decomposes at about 102 °C, with liberation of nitrogen, chlorine and oxygen. It is soluble in dilute aqueous alcohol, but insoluble in strong alcohol. This compound is a strong oxidizer and should never be stored with flammable materials.
Ammonium chlorate is a very unstable oxidizer and will decompose, sometimes violently, at room temperature. This results from the mixture of the reducing ammonium cation and the oxidizing chlorate anion. It will explode when exposed to sunlight for a few minutes. Even solutions are known to be unstable. Because of the dangerous nature of this salt it should only be kept in solution when needed, and never be allowed to crystallize.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
with the formula NH4ClO3.
It is obtained by neutralizing chloric acid
Chloric acid
Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid and oxidizing agent....
with either ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
or ammonium carbonate
Ammonium carbonate
Ammonium carbonate is a commercial salt with the chemical formula 2CO3. It is used when crushed as a smelling salt. It can be crushed when needed in order to revive someone who has fainted...
, or by precipitating barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
, strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
or calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
chlorates with ammonium carbonate or ammonium sulfate, producing the respective carbonate or sulfate precipitate and an ammonium chlorate solution. Ammonium chlorate crystallizes in small needles, readily soluble in water.
On heating, ammonium chlorate decomposes at about 102 °C, with liberation of nitrogen, chlorine and oxygen. It is soluble in dilute aqueous alcohol, but insoluble in strong alcohol. This compound is a strong oxidizer and should never be stored with flammable materials.
Ammonium chlorate is a very unstable oxidizer and will decompose, sometimes violently, at room temperature. This results from the mixture of the reducing ammonium cation and the oxidizing chlorate anion. It will explode when exposed to sunlight for a few minutes. Even solutions are known to be unstable. Because of the dangerous nature of this salt it should only be kept in solution when needed, and never be allowed to crystallize.

