Absinthin
Encyclopedia
-Absinthin is a naturally produced molecule from the plant Artemisia absinthium
(Wormwood
). It constitutes one of the most bitter chemical agents responsible for Absinthe
's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not to be confused with thujone
, the psychoactive toxin found in Absinthe
.
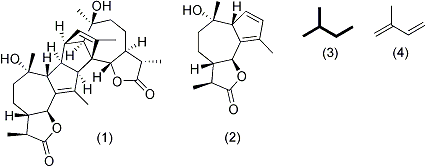
(4). The complete structure consists of two identical monomers (2) that are attached via a suspected naturally occurring Diels Alder reaction occurring at the alkenes on the 5-membered ring of the guaianolide.
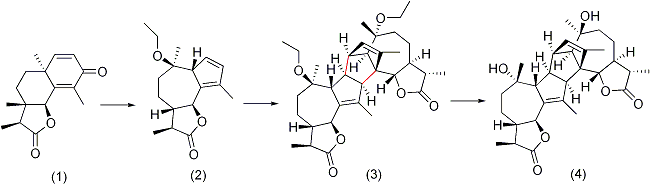
of (+)-Absinthin was conducted in 2004 by Zhang, et al. The final yield reported for the synthesis was 18.6% over a course of 10 steps originating from Santonin
(1), a commercially available reagent. The basis of the synthesis was the ring expansion of the original 6-membered carbon ring to the 7-membered ring, engendering the formation of the guaianolide monomer (2) scaffold, followed by Diels Alder coupling (3) and final stereochemical modifications resulting in (+)-Absinthin (4).
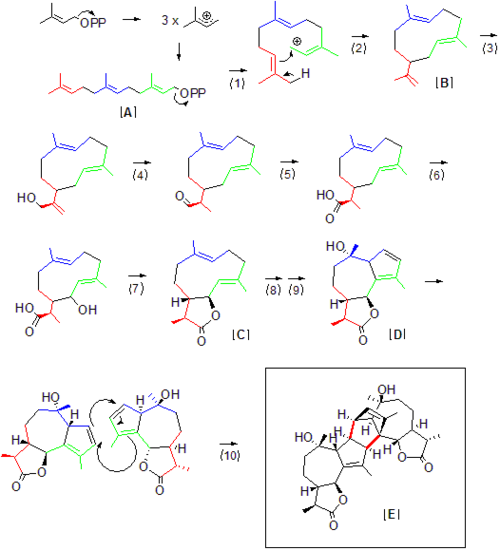
has not been elucidated, but a great portion of it can be inferred from the natural product precursors required to access Absinthin. While terpenoids like Absinthin can be said to consist of isoprene
"units," isoprene
by itself is unstable and does not react directly. Rather, the isoprene units are transferred and reacted as diphosphates. As the nomenclature for terpenes suggests, the first Absinthin precursor farnesyl diphosphate [A] contains 15 carbons, or 3 isoprene units. Diphosphate departure (1) generates a carbo-cation within the synthase, which can then be attacked by a carbon-carbon double bond at the opposing end of the molecule (2). The first stable intermediate in the biosynthesis pathway in Artemisia is likely Germacrene A [B], which has been previously identified in plant sesquiterpene pathways as a precursor to guaianolides. From there, hydroxylation (3) occurs, followed by oxidation (4) to an aldehyde directly followed by further hydroxylation (5) and formation of a carboxyl group. It is important to note the disappearance of the terminal carbon-carbon double bond after (4), as the reduction of this bond in the final product differentiates the Absinthin monomer from other Germacrene A downstream products. This reduction does not necessarily occur at step (4), but may occur further downstream. With the carboxyl and hydroxyl group in position, the guaiano-lactone [C] formation via dehydration (7) can occur, as proposed for a general guaianolide pathway. Formation of the Absinthin sesquiterpene guaianolide monomer [D] from hydroxylation and double bond rearrangement (8,9) is then postulated to directly precede dimerization to Absinthin [E] via a naturally occurring Diels-Alder reaction [10], which is likely facilitated by the associated synthase even though the reaction itself can occur in good yields spontaneously, albeit slower than typical natural product biosynthesis.
 While no synthases specific to Artemisia absinthium
While no synthases specific to Artemisia absinthium
have been sufficiently isolated to recreate this particular sesquiterpene formation in vitro, the general reaction scheme presented here portrays a likely scenario for Absinthin biosynthesis through the use of terpene intermediates utilized in the biosynthesis of Germacrene A, another sesquiterpene lactone. Enzymatic analogs from terpene biosynthesis which help rationalize the above proposed numbered biosynthetic steps are as follows:
Artemisia absinthium
Artemisia absinthium is a species of wormwood, native to temperate regions of Eurasia and northern Africa....
(Wormwood
Artemisia absinthium
Artemisia absinthium is a species of wormwood, native to temperate regions of Eurasia and northern Africa....
). It constitutes one of the most bitter chemical agents responsible for Absinthe
Absinthe
Absinthe is historically described as a distilled, highly alcoholic beverage. It is an anise-flavoured spirit derived from herbs, including the flowers and leaves of the herb Artemisia absinthium, commonly referred to as "grande wormwood", together with green anise and sweet fennel...
's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not to be confused with thujone
Thujone
Thujone is a ketone and a monoterpene that occurs naturally in two diastereomeric forms: -α-thujone and -β-thujone. It has a menthol odor. Even though it is best known as a chemical compound in the spirit absinthe, recent tests show absinthe contains only small quantities of thujone, and may or may...
, the psychoactive toxin found in Absinthe
Absinthe
Absinthe is historically described as a distilled, highly alcoholic beverage. It is an anise-flavoured spirit derived from herbs, including the flowers and leaves of the herb Artemisia absinthium, commonly referred to as "grande wormwood", together with green anise and sweet fennel...
.
Chemical Structure
Absinthin's (1) complex structure is classified as a sesquiterpene lactone, meaning it belongs to a large category of natural products chemically derived from 5-carbon "building blocks" (3) derived from isopreneIsoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
(4). The complete structure consists of two identical monomers (2) that are attached via a suspected naturally occurring Diels Alder reaction occurring at the alkenes on the 5-membered ring of the guaianolide.
Total synthesis
Total synthesisTotal synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of (+)-Absinthin was conducted in 2004 by Zhang, et al. The final yield reported for the synthesis was 18.6% over a course of 10 steps originating from Santonin
Santonin
Santonin is a drug which was widely used in the past as an anthelminthic, a drug that expels parasitic worms from the body, by either killing or stunning them. Santonin was formerly listed in U.S...
(1), a commercially available reagent. The basis of the synthesis was the ring expansion of the original 6-membered carbon ring to the 7-membered ring, engendering the formation of the guaianolide monomer (2) scaffold, followed by Diels Alder coupling (3) and final stereochemical modifications resulting in (+)-Absinthin (4).

Biosynthesis
The full biosynthesis of Absinthin in Artemisia absinthiumArtemisia absinthium
Artemisia absinthium is a species of wormwood, native to temperate regions of Eurasia and northern Africa....
has not been elucidated, but a great portion of it can be inferred from the natural product precursors required to access Absinthin. While terpenoids like Absinthin can be said to consist of isoprene
Isoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
"units," isoprene
Isoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
by itself is unstable and does not react directly. Rather, the isoprene units are transferred and reacted as diphosphates. As the nomenclature for terpenes suggests, the first Absinthin precursor farnesyl diphosphate [A] contains 15 carbons, or 3 isoprene units. Diphosphate departure (1) generates a carbo-cation within the synthase, which can then be attacked by a carbon-carbon double bond at the opposing end of the molecule (2). The first stable intermediate in the biosynthesis pathway in Artemisia is likely Germacrene A [B], which has been previously identified in plant sesquiterpene pathways as a precursor to guaianolides. From there, hydroxylation (3) occurs, followed by oxidation (4) to an aldehyde directly followed by further hydroxylation (5) and formation of a carboxyl group. It is important to note the disappearance of the terminal carbon-carbon double bond after (4), as the reduction of this bond in the final product differentiates the Absinthin monomer from other Germacrene A downstream products. This reduction does not necessarily occur at step (4), but may occur further downstream. With the carboxyl and hydroxyl group in position, the guaiano-lactone [C] formation via dehydration (7) can occur, as proposed for a general guaianolide pathway. Formation of the Absinthin sesquiterpene guaianolide monomer [D] from hydroxylation and double bond rearrangement (8,9) is then postulated to directly precede dimerization to Absinthin [E] via a naturally occurring Diels-Alder reaction [10], which is likely facilitated by the associated synthase even though the reaction itself can occur in good yields spontaneously, albeit slower than typical natural product biosynthesis.

Artemisia absinthium
Artemisia absinthium is a species of wormwood, native to temperate regions of Eurasia and northern Africa....
have been sufficiently isolated to recreate this particular sesquiterpene formation in vitro, the general reaction scheme presented here portrays a likely scenario for Absinthin biosynthesis through the use of terpene intermediates utilized in the biosynthesis of Germacrene A, another sesquiterpene lactone. Enzymatic analogs from terpene biosynthesis which help rationalize the above proposed numbered biosynthetic steps are as follows:
- Farnesyl diphosphate departure via a generic sesquiterpene synthase
- Ring closure via a generic sesquiterpene synthase (as for #1)
- Hydroxlation of terminal allylic carbon via Germacrene A hydroxylase, a cytochrome P450 enzyme.
- Oxidation of alcohol to aldol, via -germacrene A hydroxylase.
- Hydroxylation of alcohol to carboxyl group, via Germacrene A hydroxylase.
- NADPH-mediated hydroxlation of allylic carbon via a postulated hydroxylation to precede lactone ring closure
- Lactone formation/ring closure
- Hydroxylation at carbon-carbon tertiary double bond.
- Additional 5-membered ring formation/cyclization
- Diels-Alder coupling via an unidentified enzyme in Artemisia absinthium.