ANTH domain
Encyclopedia
The ANTH domain is a membrane binding domain that shows weak specificity for PtdIns(4,5)P2. It was found in AP180
(a.k.a. CALM) endocytotic accessory protein that has been implicated in the formation of clathrin-coated pits. The domain is involved in phosphatidylinositol 4,5-bisphosphate binding and is a universal adaptor for nucleation of clathrin coats.
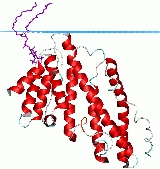
Its structure is a solenoid of 9 helices. The PtdIns(4,5)P2 binding residues are spread over several helices at the tip of the structure. The PtdIns(4,5)P2 binding sequence is Kx9Kx(K/R)(H/Y).
An ANTH domain is also found in HIP1 and HIP1R
, and the PtdIns(4,5)P2 binding sequence is conserved. More information is found on endocytosis.org.
Ap180
AP180 is a protein that plays an important role in clathrin-mediated endocytosis of synaptic vesicles. It is capable of simultaneously binding both membrane lipids and clathrin and is therefore thought to recruit clathrin to the membrane of newly invaginating vesicles...
(a.k.a. CALM) endocytotic accessory protein that has been implicated in the formation of clathrin-coated pits. The domain is involved in phosphatidylinositol 4,5-bisphosphate binding and is a universal adaptor for nucleation of clathrin coats.
Its structure is a solenoid of 9 helices. The PtdIns(4,5)P2 binding residues are spread over several helices at the tip of the structure. The PtdIns(4,5)P2 binding sequence is Kx9Kx(K/R)(H/Y).
An ANTH domain is also found in HIP1 and HIP1R
HIP1R
Huntingtin-interacting protein 1-related protein is a protein that in humans is encoded by the HIP1R gene.-Further reading:...
, and the PtdIns(4,5)P2 binding sequence is conserved. More information is found on endocytosis.org.

