Vaporization
Encyclopedia
Vaporization of an element or compound is a phase transition
from the liquid
or solid
phase to gas
phase. There are three types of vaporization: evaporation
, boiling
and sublimation.
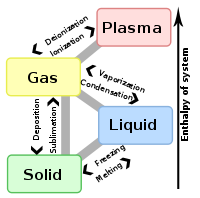 Evaporation
Evaporation
is a phase transition from the liquid phase to gas phase that occurs at temperatures below the boiling temperature
at a given pressure. Evaporation usually occurs on the surface.
Boiling
is a phase transition from the liquid phase to gas phase that occurs at or above the boiling temperature. Boiling, as opposed to evaporation, occurs below the surface.
Sublimation
is a direct phase transition from the solid phase to the gas phase, skipping the intermediate liquid phase.
The term vaporization has also been used to refer to the physical destruction of an object that is exposed to intense heat. As noted in discussions of the effects of nuclear weapons, this includes the "vaporization" of human bodies by the 1945 atomic bombings of Hiroshima and Nagasaki
and of the Marshall Island
of Elugelab
by the 1952 thermonuclear test Ivy Mike
.
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
from the liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
or solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
phase to gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
phase. There are three types of vaporization: evaporation
Evaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
, boiling
Boiling
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding environmental pressure. While below the boiling point a liquid...
and sublimation.

Evaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
is a phase transition from the liquid phase to gas phase that occurs at temperatures below the boiling temperature
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
at a given pressure. Evaporation usually occurs on the surface.
Boiling
Boiling
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding environmental pressure. While below the boiling point a liquid...
is a phase transition from the liquid phase to gas phase that occurs at or above the boiling temperature. Boiling, as opposed to evaporation, occurs below the surface.
Sublimation
Sublimation
Sublimation may refer to:* Sublimation , the change from solid to gas without entering liquid phase* Sublimation , the transformation of emotions* Sublimation , a music album by Canvas Solaris-See also:...
is a direct phase transition from the solid phase to the gas phase, skipping the intermediate liquid phase.
The term vaporization has also been used to refer to the physical destruction of an object that is exposed to intense heat. As noted in discussions of the effects of nuclear weapons, this includes the "vaporization" of human bodies by the 1945 atomic bombings of Hiroshima and Nagasaki
Atomic bombings of Hiroshima and Nagasaki
During the final stages of World War II in 1945, the United States conducted two atomic bombings against the cities of Hiroshima and Nagasaki in Japan, the first on August 6, 1945, and the second on August 9, 1945. These two events are the only use of nuclear weapons in war to date.For six months...
and of the Marshall Island
Marshall Islands
The Republic of the Marshall Islands , , is a Micronesian nation of atolls and islands in the middle of the Pacific Ocean, just west of the International Date Line and just north of the Equator. As of July 2011 the population was 67,182...
of Elugelab
Elugelab
Elugelab was an island, part of the Enewetak atoll in the Marshall Islands. It was destroyed by the world's first test of a hydrogen bomb on 1 November 1952, as part of Operation Ivy...
by the 1952 thermonuclear test Ivy Mike
Ivy Mike
Ivy Mike was the codename given to the first United States test of a thermonuclear weapon, in which a major part of the explosive yield came from nuclear fusion. It was detonated on November 1, 1952 by the United States at on Enewetak, an atoll in the Pacific Ocean, as part of Operation Ivy...
.

