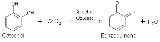
Tyrosinase
Encyclopedia
Tyrosinase also known as monophenol monooxygenase is an enzyme
that catalyses
the oxidation of phenol
s (such as tyrosine
) and is widespread in plant
s and animals. Tyrosinase is a copper-containing enzyme present in plant and animal tissues that catalyzes the production of melanin
and other pigments from tyrosine by oxidation, as in the blackening of a peeled or sliced potato
exposed to air. It is found inside melanosomes
. In humans, the tyrosinase enzyme is encoded by the TYR gene
.
, a hereditary disorder that affects one in every 17,000 people.
and dopamine
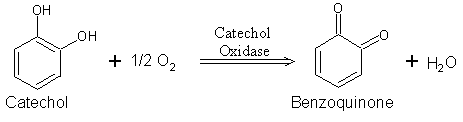
using dioxygen (O2). In the presence of catechol, benzoquinone is formed (see reaction below). Hydrogens removed from catechol combine with oxygen
to form water
.
 The substrate specificity becomes dramatically restricted in mammalian tyrosinase which utilizes only L-form of tyrosine or DOPA as substrates, and has restricted requirement for L-DOPA as cofactor.
The substrate specificity becomes dramatically restricted in mammalian tyrosinase which utilizes only L-form of tyrosine or DOPA as substrates, and has restricted requirement for L-DOPA as cofactor.
It has been suggested that there is no common tyrosinase protein structure occurring across all species. The enzymes found in plant, animal and fungal tissue frequently differ with respect to their primary structure
, size, glycosylation pattern
and activation characteristics. However, all tyrosinases have in common a binuclear type 3 copper centre
within their active site
. Here two copper atoms are each coordinated
with three histidine
residue
s.
. In humans, tyrosinase is sorted into melanosomes and the catalytically active domain of the protein resides within melanosomes. Only a small enzymatically non-essential part of the protein extends into the cytoplasm of the melanocyte
.
. The activity of tyrosinase is similar to catechol oxidase
, a related class of copper oxidase
. Tyrosinases and catechol oxidase
s are collectively termed polyphenol oxidase
s.
(MITF).
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyses
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the oxidation of phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
s (such as tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
) and is widespread in plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s and animals. Tyrosinase is a copper-containing enzyme present in plant and animal tissues that catalyzes the production of melanin
Melanin
Melanin is a pigment that is ubiquitous in nature, being found in most organisms . In animals melanin pigments are derivatives of the amino acid tyrosine. The most common form of biological melanin is eumelanin, a brown-black polymer of dihydroxyindole carboxylic acids, and their reduced forms...
and other pigments from tyrosine by oxidation, as in the blackening of a peeled or sliced potato
Potato
The potato is a starchy, tuberous crop from the perennial Solanum tuberosum of the Solanaceae family . The word potato may refer to the plant itself as well as the edible tuber. In the region of the Andes, there are some other closely related cultivated potato species...
exposed to air. It is found inside melanosomes
Melanosome
In a biological cell, a melanosome is an organelle containing melanin, the most common light-absorbing pigment found in the animal kingdom.Cells that synthesize melanins are called melanocytes, and also the retinal pigment epithelium cells, whereas cells that have merely engulfed the melanosomes...
. In humans, the tyrosinase enzyme is encoded by the TYR gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
.
Clinical significance
A mutation in the tyrosinase gene resulting in impaired tyrosinase production leads to type I oculocutaneous albinismAlbinism
Albinism is a congenital disorder characterized by the complete or partial absence of pigment in the skin, hair and eyes due to absence or defect of an enzyme involved in the production of melanin...
, a hereditary disorder that affects one in every 17,000 people.
Catalyzed reaction
Tyrosinase carries out the oxidation of phenols such as tyrosineTyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
and dopamine
Dopamine
Dopamine is a catecholamine neurotransmitter present in a wide variety of animals, including both vertebrates and invertebrates. In the brain, this substituted phenethylamine functions as a neurotransmitter, activating the five known types of dopamine receptors—D1, D2, D3, D4, and D5—and their...
using dioxygen (O2). In the presence of catechol, benzoquinone is formed (see reaction below). Hydrogens removed from catechol combine with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
to form water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
.

Tyrosinase structure
Tyrosinases have been isolated and studied from a wide variety of plant, animal and fungal species. Tyrosinases from different species are diverse in terms of their structural properties, tissue distribution and cellular location.It has been suggested that there is no common tyrosinase protein structure occurring across all species. The enzymes found in plant, animal and fungal tissue frequently differ with respect to their primary structure
Primary structure
The primary structure of peptides and proteins refers to the linear sequence of its amino acid structural units. The term "primary structure" was first coined by Linderstrøm-Lang in 1951...
, size, glycosylation pattern
Glycosylation
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule . In biology glycosylation refers to the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules...
and activation characteristics. However, all tyrosinases have in common a binuclear type 3 copper centre
Copper proteins
Copper proteins are proteins that contain one or more copper ions as prosthetic groups. The metal centres in the copper proteins can be classified into several types:...
within their active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
. Here two copper atoms are each coordinated
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
with three histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residue
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
s.
Transmembrane protein and sorting
Human tyrosinase is a single membrane spanning transmembrane proteinTransmembrane protein
A transmembrane protein is a protein that goes from one side of a membrane through to the other side of the membrane. Many TPs function as gateways or "loading docks" to deny or permit the transport of specific substances across the biological membrane, to get into the cell, or out of the cell as...
. In humans, tyrosinase is sorted into melanosomes and the catalytically active domain of the protein resides within melanosomes. Only a small enzymatically non-essential part of the protein extends into the cytoplasm of the melanocyte
Melanocyte
-External links: - "Eye: fovea, RPE" - "Integument: pigmented skin"...
.
Active site
The two copper atoms within the active site of tyrosinase enzymes interact with dioxygen to form a highly reactive chemical intermediate that then oxidizes the substrateSubstrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
. The activity of tyrosinase is similar to catechol oxidase
Catechol oxidase
Catechol oxidase is an enzyme that catalyses the oxidation of phenols such as catechol. Catechol oxidase is a copper-containing enzyme whose activity is similar to that of tyrosinase, a related class of copper oxidases....
, a related class of copper oxidase
Oxidase
An oxidase is any enzyme that catalyzes an oxidation-reduction reaction involving molecular oxygen as the electron acceptor. In these reactions, oxygen is reduced to water or hydrogen peroxide ....
. Tyrosinases and catechol oxidase
Catechol oxidase
Catechol oxidase is an enzyme that catalyses the oxidation of phenols such as catechol. Catechol oxidase is a copper-containing enzyme whose activity is similar to that of tyrosinase, a related class of copper oxidases....
s are collectively termed polyphenol oxidase
Polyphenol oxidase
Polyphenol oxidase is a tetramer which contains four atoms of copper per molecule, and binding sites for two aromatic compounds and oxygen...
s.
Gene regulation
The gene for tyrosinase is regulated by the microphthalmia-associated transcription factorMicrophthalmia-associated transcription factor
Microphthalmia-associated transcription factor is a basic helix-loop-helix leucine zipper transcription factor involved in melanocyte and osteoclast development.-Clinical significance:...
(MITF).

