
Tropospheric ozone
Encyclopedia
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
(O3) is a constituent of the troposphere
Troposphere
The troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
(it is also an important constituent of certain regions of the stratosphere
Stratosphere
The stratosphere is the second major layer of Earth's atmosphere, just above the troposphere, and below the mesosphere. It is stratified in temperature, with warmer layers higher up and cooler layers farther down. This is in contrast to the troposphere near the Earth's surface, which is cooler...
commonly known as the Ozone layer
Ozone layer
The ozone layer is a layer in Earth's atmosphere which contains relatively high concentrations of ozone . This layer absorbs 97–99% of the Sun's high frequency ultraviolet light, which is potentially damaging to the life forms on Earth...
). Photochemical and chemical reactions involving it drive many of the chemical processes that occur in the atmosphere by day and by night. At abnormally high concentrations brought about by human activities (largely incomplete combustion of fossil fuels, such as gasoline, diesel, etc.), it is a pollutant
Pollutant
A pollutant is a waste material that pollutes air, water or soil, and is the cause of pollution.Three factors determine the severity of a pollutant: its chemical nature, its concentration and its persistence. Some pollutants are biodegradable and therefore will not persist in the environment in the...
, and a constituent of smog
Smog
Smog is a type of air pollution; the word "smog" is a portmanteau of smoke and fog. Modern smog is a type of air pollution derived from vehicular emission from internal combustion engines and industrial fumes that react in the atmosphere with sunlight to form secondary pollutants that also combine...
. Many highly energetic reactions produce it, ranging from combustion to photocopying. Often laser printers will have a smell of ozone, which in high concentrations is toxic. Ozone is a powerful oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
readily reacting with other chemical compounds to make many possibly toxic oxides.
The troposphere
Troposphere
The troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
extends from the surface of the Earth
Earth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
to between 10 and 18 kilometers above the surface of the Earth
Earth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
and consists of many layers. Ozone is more concentrated above the mixing layer, or ground layer. Ground-level ozone, though less concentrated than ozone aloft, is more of a problem because of its health effects.
Tropospheric ozone is a greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
and initiates the chemical removal of methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
and other hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s from the atmosphere. Thus, its concentration affects how long these compounds remain in the air.
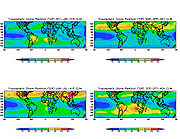
Satellite
Satellite
In the context of spaceflight, a satellite is an object which has been placed into orbit by human endeavour. Such objects are sometimes called artificial satellites to distinguish them from natural satellites such as the Moon....
s can measure tropospheric ozone.http://tes.jpl.nasa.gov/http://jwocky.gsfc.nasa.gov/ Measurements specifically of ground-level ozone require in situ monitoring technology.
Formation
The majority of tropospheric ozone formation occurs when nitrogen oxides (NOx), carbon monoxideCarbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
(CO) and volatile organic compounds (VOCs), such as xylene
Xylene
Xylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached...
, react in the atmosphere in the presence of sunlight. NOx, CO, and VOCs are called ozone precursors. Motor vehicle exhaust, industrial emissions, and chemical solvents are the major anthropogenic sources of these chemicals. Another source is windshield washer fluid. Although these precursors often originate in urban areas, winds can carry NOx hundreds of kilometers, causing ozone formation to occur in less populated regions as well. Methane, a VOC whose atmospheric concentration has increased tremendously during the last century, contributes to ozone formation but on a global scale rather than in local or regional photochemical smog episodes. In situations where this exclusion of methane from the VOC group of substances is not obvious, the term Non-Methane VOC (NMVOC) is often used.
The chemical reactions involved in tropospheric ozone formation are a series of complex cycles in which carbon monoxide and VOCs are oxidised to water vapour and carbon dioxide. The reactions involved in this process are illustrated here with CO but similar reactions occur for VOC as well. Oxidation begins with the reaction of CO with the hydroxyl radical
Hydroxyl radical
The hydroxyl radical, •OH, is the neutral form of the hydroxide ion . Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides or, in...
. The hydrogen atom formed by this reacts rapidly with oxygen to give a peroxy radical
Hydroperoxyl
The hydroperoxyl radical, also known as the perhydroxyl radical, is the protonated form of superoxide with the chemical formula HO2.-Reactivity:The superoxide anion, O2−, and the hydroperoxyl radical are in equilibrium in aqueous solution:...
HO2
-
- OH + CO → H + CO2
- H + O2 → HO2
Peroxy radicals then go on to react with NO to give NO2 which is photolysed to give atomic oxygen and through reaction with oxygen a molecule of ozone:
-
- HO2 + NO → OH + NO2
- NO2 + hν → NO + O
- O + O2 + N2 → O3 + N2
The net effect of these reactions is:
-
- CO + 2O2 → CO2 + O3
This cycle involving HOx and NOx is terminated by the reaction of OH with NO2 to form nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
or by the reaction of peroxy radicals with each other to form peroxides. The chemistry involving VOCs is much more complex but the same reaction of peroxy radicals oxidizing NO to NO2 is the critical step leading to ozone formation.
Health effects
Ozone is known to have the following health effects at concentrations common in urban air:- Irritation of the respiratory systemRespiratory systemThe respiratory system is the anatomical system of an organism that introduces respiratory gases to the interior and performs gas exchange. In humans and other mammals, the anatomical features of the respiratory system include airways, lungs, and the respiratory muscles...
, causing coughing, throat irritation, and/or an uncomfortable sensation in the chest. - Reduced lungLungThe lung is the essential respiration organ in many air-breathing animals, including most tetrapods, a few fish and a few snails. In mammals and the more complex life forms, the two lungs are located near the backbone on either side of the heart...
function, making it more difficult to breathe deeply and vigorously. Breathing may become more rapid and more shallow than normal, and a person's ability to engage in vigorous activities may be limited. - Aggravation of asthmaAsthmaAsthma is the common chronic inflammatory disease of the airways characterized by variable and recurring symptoms, reversible airflow obstruction, and bronchospasm. Symptoms include wheezing, coughing, chest tightness, and shortness of breath...
. When ozone levels are high, more people with asthma have attacks that require a doctor's attention or use of medication. One reason this happens is that ozone makes people more sensitive to allergenAllergenAn allergen is any substance that can cause an allergy. In technical terms, an allergen is a non-parasitic antigen capable of stimulating a type-I hypersensitivity reaction in atopic individuals....
s, which in turn trigger asthma attacks. - Increased susceptibility to respiratory infectionsUpper respiratory tract infectionUpper respiratory tract infections are the illnesses caused by an acute infection which involves the upper respiratory tract: nose, sinuses, pharynx or larynx...
. - Inflammation and damage to the lining of the lungs. Within a few days, the damaged cells are shed and replaced much like the skin peels after a sunburn. Animal studies suggest that if this type of inflammation happens repeatedly over a long time period (months, years, a lifetime), lung tissue may become permanently scarred, resulting in permanent loss of lung function and a lower quality of life.
A statistical study of 95 large urban communities in the United States found significant association between ozone levels and premature death. The study estimated that a one-third reduction in urban ozone concentrations would save roughly 4000 lives per year (Bell et al., 2004).
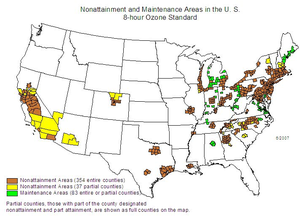
Problem areas
United States Environmental Protection Agency
The U.S. Environmental Protection Agency is an agency of the federal government of the United States charged with protecting human health and the environment, by writing and enforcing regulations based on laws passed by Congress...
has developed an Air Quality index to help explain air pollution levels to the general public. 8-hour average ozone mole fractions of 85 to 104 nmol/mol are described as "Unhealthy for Sensitive Groups", 105 nmol/mol to 124 nmol/mol as "unhealthy" and 125 nmol/mol to 404 nmol/mol as "very unhealthy" http://www.airnow.gov/index.cfm?action=health2.smog1#4. The EPA has designated over 300 counties of the United States, clustered around the most heavily populated areas (especially in California and the Northeast), as failing to comply with the National Ambient Air Quality Standards
National Ambient Air Quality Standards
The National Ambient Air Quality Standards are standards established by the United States Environmental Protection Agency under authority of the Clean Air Act that apply for outdoor air throughout the country...
.
See also
- Canada-Wide Standards for particulate matter (PM2.5) and ozone http://www.ccme.ca/assets/pdf/pmozone_standard_e.pdf (pdf)
- National Ambient Air Quality StandardsNational Ambient Air Quality StandardsThe National Ambient Air Quality Standards are standards established by the United States Environmental Protection Agency under authority of the Clean Air Act that apply for outdoor air throughout the country...
(USA) - OzoneOzoneOzone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
- Photochemical smog
- TroposphereTroposphereThe troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
- Criteria air contaminantsCriteria air contaminantsCriteria air contaminants , or criteria pollutants, are a set of air pollutants that cause smog, acid rain and other health hazards. The laws and regulations of different polities may define different sets. CACs are typically emitted from many sources in industry, mining, transportation,...
- Tropospheric ozone depletion eventsTropospheric ozone depletion eventsDuring springtime in the polar regions, unique photochemistry converts inert halide salt ions into reactive halogen species that deplete ozone in the boundary layer to near zero levels. Since their discovery in the late 1980s, research on these ozone depletion events has shown the central role...
External links
- The European Environment Agency's near real-time ozone map (ozoneweb)
- U.S. Environmental Protection Agency Ozone Information
- U.S. Environmental Protection Agency Live Ozone Map
- U.S. Environmental Protection Agency Ozone Regulation Information
- University Corporation for Atmospheric Research on ozone pollution
- Total Ozone Mapping Spectrometer (satellite monitoring)
- WHO-Europe reports: Health Aspects of Air Pollution (2003) (PDF) and "Answer to follow-up questions from CAFE (2004) (PDF)
- NASA's Ozone Resource Page

