
Thermodynamic processes
Encyclopedia
A thermodynamic process may be defined as the energetic development of a thermodynamic system
proceeding from an initial state to a final state. Paths through the space of thermodynamic variables are often specified by holding certain thermodynamic variables constant. A state function
is a thermodynamic variable which depends only on the current state of the system, not the path taken to reach that state. Conversely a process function
does depend on the path.
s. In the example, four processes are shown. Each process has a well-defined start and end point in the pressure-volume state space
. In this particular example, processes 1 and 3 are isothermal, whereas processes 2 and 4 are isochoric
. The PV diagram is a particularly useful visualization of a process, because the area under the curve of a process is the amount of work
done by the system during that process. Thus work is considered to be a process variable
, as its exact value depends on the particular path taken between the start and end points of the process. Similarly, heat
may be transferred during a process, and it too is a process variable. In contrast, pressure and volume (as well as numerous other properties) are considered state variables because their values depend only on the position of the start and end points, not the particular path between them.
pair.
–particle number
conjugate pair, which is concerned with the transfer of energy via this transfer of particles.
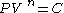 ,
,
where P is the pressure, V is volume, n is any real number
(the "polytropic index"), and C is a constant. This equation can be used to accurately characterize processes of certain system
s, notably the compression or expansion
of a gas
, but in some cases, liquid
s and solid
s.
(so the system remains in quasistatic equilibrium
), in which case the process is typically reversible
.
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
proceeding from an initial state to a final state. Paths through the space of thermodynamic variables are often specified by holding certain thermodynamic variables constant. A state function
State function
In thermodynamics, a state function, function of state, state quantity, or state variable is a property of a system that depends only on the current state of the system, not on the way in which the system acquired that state . A state function describes the equilibrium state of a system...
is a thermodynamic variable which depends only on the current state of the system, not the path taken to reach that state. Conversely a process function
Process function
A process function, process quantity, or a path function is a physical quantity that describes the transition of a system from an equilibrium state to another equilibrium state. As an example, mechanical work and heat are process quantities because they describe quantitatively the transition...
does depend on the path.
Overview
A thermodynamic process can be visualized by graphically plotting the changes to the system's state variableState variable
A state variable is one of the set of variables that describe the "state" of a dynamical system. Intuitively, the state of a system describes enough about the system to determine its future behaviour...
s. In the example, four processes are shown. Each process has a well-defined start and end point in the pressure-volume state space
State space
In the theory of discrete dynamical systems, a state space is a directed graph where each possible state of a dynamical system is represented by a vertex, and there is a directed edge from a to b if and only if ƒ = b where the function f defines the dynamical system.State spaces are...
. In this particular example, processes 1 and 3 are isothermal, whereas processes 2 and 4 are isochoric
Isochoric process
An isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant...
. The PV diagram is a particularly useful visualization of a process, because the area under the curve of a process is the amount of work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
done by the system during that process. Thus work is considered to be a process variable
Process variable
A process variable, process value or process parameter is the current status of a process under control. An example of this would be the temperature of a furnace. The current temperature is called the process variable, while the desired temperature is known as the set-point.Measurement of process...
, as its exact value depends on the particular path taken between the start and end points of the process. Similarly, heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
may be transferred during a process, and it too is a process variable. In contrast, pressure and volume (as well as numerous other properties) are considered state variables because their values depend only on the position of the start and end points, not the particular path between them.
Conjugate variable processes
It is often useful to group processes into pairs, in which each variable held constant is one member of a conjugateConjugate variables (thermodynamics)
In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables such as temperature/entropy or pressure/volume. In fact all thermodynamic potentials are expressed in terms of conjugate pairs....
pair.
Pressure - volume
The pressure-volume conjugate pair is concerned with the transfer of mechanical or dynamic energy as the result of work.- An isobaric processIsobaric processAn isobaric process is a thermodynamic process in which the pressure stays constant. The term derives from the Greek isos, , and barus,...
occurs at constant pressure. An example would be to have a movable piston in a cylinder, so that the pressure inside the cylinder is always at atmospheric pressure, although it is isolated from the atmosphere. In other words, the system is dynamically connected, by a movable boundary, to a constant-pressure reservoir.
- An isochoric processIsochoric processAn isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant...
is one in which the volume is held constant, meaning that the work done by the system will be zero. It follows that, for the simple system of two dimensions, any heat energy transferred to the system externally will be absorbed as internal energy. An isochoric process is also known as an isometric process or an isovolumetric process. An example would be to place a closed tin can containing only air into a fire. To a first approximation, the can will not expand, and the only change will be that the gas gains internal energy, as evidenced by its increase in temperature and pressure. Mathematically, . We may say that the system is dynamically insulated, by a rigid boundary, from the environment.
. We may say that the system is dynamically insulated, by a rigid boundary, from the environment.
Temperature - entropy
The temperature-entropy conjugate pair is concerned with the transfer of thermal energy as the result of heating.- An isothermal processIsothermal processAn isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir...
occurs at a constant temperature. An example would be to have a system immersed in a large constant-temperature bath. Any work energy performed by the system will be lost to the bath, but its temperature will remain constant. In other words, the system is thermally connected, by a thermally conductive boundary to a constant-temperature reservoir.
- An adiabatic processAdiabatic processIn thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
is a process in which there is no energy added or subtracted from the system by heating or cooling. For a reversible process, this is identical to an isentropic process. We may say that the system is thermally insulated from its environment and that its boundary is a thermal insulator. If a system has an entropy which has not yet reached its maximum equilibrium value, the entropy will increase even though the system is thermally insulated. Under certain conditions two states of a system may be considered adiabatically accesisbleAdiabatic accessibilityAdiabatic accessibility denotes a certain relation between two equilibrium states of a thermodynamic system . The concept was coined by Constantin Carathéodory in 1909 and taken up 90 years later by Elliott Lieb and J. Yngvason in their axiomatic approach to the foundations of thermodynamics . It...
.
- An isentropic processIsentropic processIn thermodynamics, an isentropic process or isoentropic process is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the...
occurs at a constant entropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
. For a reversible process this is identical to an adiabatic process. If a system has an entropy which has not yet reached its maximum equilibrium value, a process of cooling may be required to maintain that value of entropy.
Chemical potential - particle number
The processes above have all implicitly assumed that the boundaries are also impermeable to particles. We may assume boundaries that are both rigid and thermally insulating, but are permeable to one or more types of particle. Similar considerations then hold for the chemical potentialChemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
–particle number
Particle number
The particle number of a thermodynamic system, conventionally indicated with the letter N, is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is conjugate to the chemical potential. Unlike most physical quantities, particle...
conjugate pair, which is concerned with the transfer of energy via this transfer of particles.
- In a constant chemical potential process the system is particle-transfer connected, by a particle-permeable boundary, to a constant-µ reservoir.
- In a constant particle number process there is no energy added or subtracted from the system by particle transfer. We may say that the system is particle-transfer-insulated from its environment by a boundary that is impermeable to particles.
Thermodynamic potentials
Any of the thermodynamic potentials may be held constant during a process. For example:- An isenthalpic processIsenthalpic processAn isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy, H; or specific enthalpy, h....
introduces no change in enthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
in the system.
Polytropic processes
A polytropic process is a thermodynamic process that obeys the relation: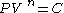 ,
,where P is the pressure, V is volume, n is any real number
Real number
In mathematics, a real number is a value that represents a quantity along a continuum, such as -5 , 4/3 , 8.6 , √2 and π...
(the "polytropic index"), and C is a constant. This equation can be used to accurately characterize processes of certain system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
s, notably the compression or expansion
Thermal expansion
Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
of a gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
, but in some cases, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
s and solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
s.
Quasistatic process
A quasistatic process is an idealized model of a thermodynamic process that happens infinitely slowly. It is important to note that no real process is quasistatic. In practice, such processes can only be approximated by performing them infinitesimally slowly. A quasistatic process often ensures that the system will go through a sequence of states that are infinitesimally close to equilibriumThermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
(so the system remains in quasistatic equilibrium
Quasistatic equilibrium
Quasistatic equilibrium is the quasi-balanced state of a thermodynamic system near to thermodynamic equilibrium in some sense or degree...
), in which case the process is typically reversible
Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
.
See also
- Kalina cycleKalina cycleThe Kalina cycle is a thermodynamic process for converting thermal energy into usable mechanical power.It uses a solution of 2 fluids with different boiling points for its working fluid. Since the solution boils over a range of temperatures as in distillation, more of the heat can be extracted...
- Phase transitionPhase transitionA phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....

