
Tetraoxygen
Encyclopedia
The tetraoxygen molecule (O4), also called oxozone, was first predicted in 1924 by Gilbert N. Lewis
, who proposed it as an explanation for the failure of liquid oxygen
to obey Curie's law
. Today it seems Lewis was off, but not by much: computer simulations indicate that although there are no stable O4 molecules in liquid oxygen, O2 molecules do tend to associate in pairs with antiparallel spin
s, forming transient O4 units. In 1999, researchers thought that solid oxygen
existed in its ε-phase (at pressures above 10 GPa) as O4. However, in 2006, it was shown by X-ray crystallography
that this stable phase
known as ε oxygen or red oxygen is in fact . Nevertheless, tetraoxygen has been detected as a short-lived chemical species
in mass spectrometry
experiments.
Absorption bands of the O4 molecule e.g. at 360, 477 and 577 nm are frequently used to do aerosol inversions in atmospheric optical absorption spectroscopy
. Due to the known distribution of O2 and therefore also O4, O4 slant column densities can be used to retrieve aerosol profiles which can then be used again in radiative transfer models to model light paths.
, and a "pinwheel" with three oxygen atoms surrounding a central one in a trigonal planar formation similar to boron trifluoride
. It was previously pointed out that the "pinwheel" O4 molecule should be the natural continuation of the isoelectronic series BO33-
, CO32-
, NO3-
, and analogous to SO3
; that observation served as the basis for the mentioned theoretical calculations.
In 2001, a team at the University of Rome La Sapienza
conducted a neutralization-reionization mass spectrometry experiment to investigate the structure of free O4 molecules. Their results did not agree with either of the two proposed molecular structures, but they did agree with a complex
between two O2 molecules, one in the ground state
and the other in a specific excited state
.
Gilbert N. Lewis
Gilbert Newton Lewis was an American physical chemist known for the discovery of the covalent bond , his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and...
, who proposed it as an explanation for the failure of liquid oxygen
Liquid oxygen
Liquid oxygen — abbreviated LOx, LOX or Lox in the aerospace, submarine and gas industries — is one of the physical forms of elemental oxygen.-Physical properties:...
to obey Curie's law
Curie's law
In a paramagnetic material the magnetization of the material is directly proportional to an applied magnetic field. However, if the material is heated, this proportionality is reduced: for a fixed value of the field, the magnetization is inversely proportional to temperature...
. Today it seems Lewis was off, but not by much: computer simulations indicate that although there are no stable O4 molecules in liquid oxygen, O2 molecules do tend to associate in pairs with antiparallel spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
s, forming transient O4 units. In 1999, researchers thought that solid oxygen
Solid oxygen
Solid oxygen forms at normal atmospheric pressure at a temperature below 54.36 K . Solid oxygen O2, like liquid oxygen, is a clear substance with a light sky-blue color caused by absorption in the red....
existed in its ε-phase (at pressures above 10 GPa) as O4. However, in 2006, it was shown by X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
that this stable phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
known as ε oxygen or red oxygen is in fact . Nevertheless, tetraoxygen has been detected as a short-lived chemical species
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
in mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
experiments.
Absorption bands of the O4 molecule e.g. at 360, 477 and 577 nm are frequently used to do aerosol inversions in atmospheric optical absorption spectroscopy
Absorption spectroscopy
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a...
. Due to the known distribution of O2 and therefore also O4, O4 slant column densities can be used to retrieve aerosol profiles which can then be used again in radiative transfer models to model light paths.
Free molecule
Theoretical calculations have predicted the existence of metastable O4 molecules with two different shapes: a "puckered" square like cyclobutaneCyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
, and a "pinwheel" with three oxygen atoms surrounding a central one in a trigonal planar formation similar to boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
. It was previously pointed out that the "pinwheel" O4 molecule should be the natural continuation of the isoelectronic series BO33-
Borate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
, CO32-
Carbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
, NO3-
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
, and analogous to SO3
Sulfur trioxide
Sulfur trioxide is the chemical compound with the formula SO3. In the gaseous form, this species is a significant pollutant, being the primary agent in acid rain. It is prepared on massive scales as a precursor to sulfuric acid.-Structure and bonding:Gaseous SO3 is a trigonal planar molecule of...
; that observation served as the basis for the mentioned theoretical calculations.
| Theoretical structures of metastable O4. | |
 |
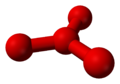 |
In 2001, a team at the University of Rome La Sapienza
University of Rome La Sapienza
The Sapienza University of Rome, officially Sapienza – Università di Roma, formerly known as Università degli studi di Roma "La Sapienza", is a coeducational, autonomous state university in Rome, Italy...
conducted a neutralization-reionization mass spectrometry experiment to investigate the structure of free O4 molecules. Their results did not agree with either of the two proposed molecular structures, but they did agree with a complex
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
between two O2 molecules, one in the ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
and the other in a specific excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
.
See also
- TetranitrogenTetranitrogenAs reported in the January 18, 2002 edition of Science at the Sapienza University of Rome, Fulvio Cacace and his colleagues created a new nitrogen molecule known as tetranitrogen, with the structure N4...
(N4) - Solid oxygenSolid oxygenSolid oxygen forms at normal atmospheric pressure at a temperature below 54.36 K . Solid oxygen O2, like liquid oxygen, is a clear substance with a light sky-blue color caused by absorption in the red....
- Liquid oxygenLiquid oxygenLiquid oxygen — abbreviated LOx, LOX or Lox in the aerospace, submarine and gas industries — is one of the physical forms of elemental oxygen.-Physical properties:...
- OxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...

