Tetrafluoromethane
Encyclopedia
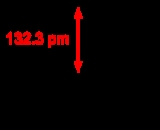
Tetrafluoromethane, also known as carbon tetrafluoride, is the simplest fluorocarbon
(CF4). It has a very high bond strength due to the nature of the carbon–fluorine bond
. It can also be classified as a haloalkane
or halomethane
. Because of the multiple carbon–fluorine bonds, and the highest electronegativity
of fluorine
, the carbon in tetrafluoromethane has a significant positive partial charge
which strengthens and shortens the four carbon–fluorine bonds by providing additional ionic
character. Tetrafluoromethane is a potent greenhouse gas
.
. Additionally, they strengthen as more carbon–fluorine bonds are added to the same carbon. In the one carbon organofluorine compounds represented by molecules of fluoromethane
, difluoromethane
, trifluoromethane, and tetrafluoromethane, the carbon–fluorine bonds are strongest in tetrafluoromethane. This effect is due to the increased coulombic attractions between the fluorine atoms and the carbon because the carbon has a positive partial charge
of 0.76.
is a coproduct. It was first reported in 1926. It can also be prepared by the fluorination of carbon dioxide
, carbon monoxide
or phosgene
with sulfur tetrafluoride
. Commercially it is manufactured by the reaction of fluorine
with dichlorodifluoromethane
or chlorotrifluoromethane
; it is also produced during the electrolysis
of metal fluoride
s MF, MF2 using a carbon electrode.
Although it can be made from myriad precursors and fluorine, elemental fluorine is expensive and difficult to handle. Consequently CF4 is prepared on an industrial scale using hydrogen fluoride
:
with fluorine.
s. Thermal decomposition or combustion of CF4 produces toxic gases (carbonyl fluoride
and carbon monoxide
) and in the presence of water will also yield hydrogen fluoride
.
It is very slightly soluble in water (about 20 mg.l−1), but miscible with organic solvents.
. It is used in electronics
microfabrication
alone or in combination with oxygen
as a plasma etchant
for silicon
, silicon dioxide
, and silicon nitride
.
that contributes to the greenhouse effect
. It is very stable, has an atmospheric lifespan of 50,000 years, and a high greenhouse warming potential of 6500 (CO2
has a factor of 1); however, the low amount in the atmosphere restricts the overall radiative forcing
effect.
Although structurally similar to chlorofluorocarbons (CFCs), tetrafluoromethane does not deplete the ozone layer
. This is because the depletion is caused by the chlorine atoms in CFCs, which dissociate when struck by UV radiation. Carbon-fluorine bonds are stronger and less likely to dissociate.
According to Guinness World Records
Tetrafluoromethane is the most persistent greenhouse gas.
Due to its density, tetrafluoromethane can displace air, creating an asphyxiation hazard in inadequately ventilated areas.
Fluorocarbon
Fluorocarbons, sometimes referred to as perfluorocarbons or PFCs, are organofluorine compounds that contain only carbon and fluorine bonded together in strong carbon–fluorine bonds. Fluoroalkanes that contain only single bonds are more chemically and thermally stable than alkanes...
(CF4). It has a very high bond strength due to the nature of the carbon–fluorine bond
Carbon–fluorine bond
The carbon–fluorine bond is a bond between carbon and fluorine that is a component of all organofluorine compounds. It is the strongest single bond in organic chemistry—and relatively short—due to its partial ionic character. The bond also strengthens and shortens as more fluorines are...
. It can also be classified as a haloalkane
Haloalkane
The haloalkanes are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and...
or halomethane
Halomethane
Halomethane compounds are derivatives of methane with one or more of the hydrogen atoms replaced with halogen atoms . Halomethanes are both naturally occurring, especially in marine environments, and man-made, most notably as refrigerants, solvents, propellants, and fumigants...
. Because of the multiple carbon–fluorine bonds, and the highest electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
, the carbon in tetrafluoromethane has a significant positive partial charge
Partial charge
A partial charge is a charge with an absolute value of less than one elementary charge unit .-Partial atomic charges:...
which strengthens and shortens the four carbon–fluorine bonds by providing additional ionic
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
character. Tetrafluoromethane is a potent greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
.
Bonding
Carbon–fluorine bonds are the strongest in organic chemistryOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. Additionally, they strengthen as more carbon–fluorine bonds are added to the same carbon. In the one carbon organofluorine compounds represented by molecules of fluoromethane
Fluoromethane
Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane plus fluorine, minus a hydrogen...
, difluoromethane
Difluoromethane
Difluoromethane, also called HFC-32 or R-32, is an organic compound of the dihalogenoalkane variety. It is based on methane, except that two of the four hydrogen atoms have been replaced by fluorine atoms...
, trifluoromethane, and tetrafluoromethane, the carbon–fluorine bonds are strongest in tetrafluoromethane. This effect is due to the increased coulombic attractions between the fluorine atoms and the carbon because the carbon has a positive partial charge
Partial charge
A partial charge is a charge with an absolute value of less than one elementary charge unit .-Partial atomic charges:...
of 0.76.
Preparation
Tetrafluoromethane is the product when any carbon compound, including carbon itself, is burns in an atmosphere of fluorine. With hydrocarbons, hydrogen fluorideHydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
is a coproduct. It was first reported in 1926. It can also be prepared by the fluorination of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
or phosgene
Phosgene
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy as a chemical weapon during World War I. It is also a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In low concentrations, its odor resembles...
with sulfur tetrafluoride
Sulfur tetrafluoride
Sulfur tetrafluoride is the chemical compound with the formula SF4. This species exists as a gas at standard conditions. It is a corrosive species that releases dangerous HF upon exposure to water or moisture...
. Commercially it is manufactured by the reaction of fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
with dichlorodifluoromethane
Dichlorodifluoromethane
Dichlorodifluoromethane , is a colorless gas, and usually sold under the brand name Freon-12, is a chlorofluorocarbon halomethane , used as a refrigerant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in the United States along with many other...
or chlorotrifluoromethane
Chlorotrifluoromethane
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive chlorofluorocarbon and also a mixed halomethane. It is used as a refrigerant, however, due to concerns about its ozone-depleting potential, its use has been phased out due to the Montreal Protocol.-Physical...
; it is also produced during the electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of metal fluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
s MF, MF2 using a carbon electrode.
Although it can be made from myriad precursors and fluorine, elemental fluorine is expensive and difficult to handle. Consequently CF4 is prepared on an industrial scale using hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
:
- CCl2F2 + 2 HF → CF4 + 2 HCl
Laboratory synthesis
Tetrafluoromethane can be prepared in the laboratory by the reaction of silicon carbideSilicon carbide
Silicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
with fluorine.
- SiC + 2 F2 → CF4 + Si
Reactions
Tetrafluoromethane, like other fluorocarbons, is very stable due to the strength of its carbon-fluorine bonds. The bonds in tetrafluoromethane have a bonding energy of 515 kJ.mol−1. As a result, it is inert to acids and hydroxides. However, it reacts explosively with alkali metalAlkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
s. Thermal decomposition or combustion of CF4 produces toxic gases (carbonyl fluoride
Carbonyl fluoride
Carbonyl fluoride is the chemical compound with the formula COF2. This gas, like its analog phosgene, is highly toxic. The molecule is planar with C2v symmetry.-Safety:...
and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
) and in the presence of water will also yield hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
.
It is very slightly soluble in water (about 20 mg.l−1), but miscible with organic solvents.
Uses
Tetrafluoromethane is sometimes used as a low temperature refrigerantRefrigerant
A refrigerant is a substance used in a heat cycle usually including, for enhanced efficiency, a reversible phase change from a liquid to a gas. Traditionally, fluorocarbons, especially chlorofluorocarbons, were used as refrigerants, but they are being phased out because of their ozone depletion...
. It is used in electronics
Electronics
Electronics is the branch of science, engineering and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits, and associated passive interconnection technologies...
microfabrication
Microfabrication
Microfabrication is the term that describes processes of fabrication of miniature structures, of micrometre sizes and smaller. Historically the earliest microfabrication processes were used for integrated circuit fabrication, also known as "semiconductor manufacturing" or "semiconductor device...
alone or in combination with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
as a plasma etchant
Plasma etching
Plasma etching is a form of plasma processing used to fabricate integrated circuits. It involves a high-speed stream of glow discharge of an appropriate gas mixture being shot at a sample. The plasma source, known as etch species, can be either charged or neutral...
for silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, silicon dioxide
Silicon dioxide
The chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity...
, and silicon nitride
Silicon nitride
Silicon nitride is a chemical compound of silicon and nitrogen. If powdered silicon is heated between 1300° and 1400°C in an atmosphere of nitrogen, trisilicon tetranitride, Si3N4, is formed. The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen...
.
Environmental effects
Tetrafluoromethane is a potent greenhouse gasGreenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
that contributes to the greenhouse effect
Greenhouse effect
The greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface, energy is transferred to the surface and the lower atmosphere...
. It is very stable, has an atmospheric lifespan of 50,000 years, and a high greenhouse warming potential of 6500 (CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
has a factor of 1); however, the low amount in the atmosphere restricts the overall radiative forcing
Radiative forcing
In climate science, radiative forcing is generally defined as the change in net irradiance between different layers of the atmosphere. Typically, radiative forcing is quantified at the tropopause in units of watts per square meter. A positive forcing tends to warm the system, while a negative...
effect.
Although structurally similar to chlorofluorocarbons (CFCs), tetrafluoromethane does not deplete the ozone layer
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
. This is because the depletion is caused by the chlorine atoms in CFCs, which dissociate when struck by UV radiation. Carbon-fluorine bonds are stronger and less likely to dissociate.
According to Guinness World Records
Guinness World Records
Guinness World Records, known until 2000 as The Guinness Book of Records , is a reference book published annually, containing a collection of world records, both human achievements and the extremes of the natural world...
Tetrafluoromethane is the most persistent greenhouse gas.
Health risks
Depending on the concentration, inhalation of tetrafluoromethane can cause headaches, nausea, dizziness and damage to the cardiovascular system (mainly the heart). Long-term exposure can cause severe heart damage.Due to its density, tetrafluoromethane can displace air, creating an asphyxiation hazard in inadequately ventilated areas.
External links
- National Pollutant Inventory - Fluoride and compounds fact sheet
- Data from Air Liquide
- Vapor pressure graph at Air Liquide
- MSDS at Oxford University
- Protocol for measurement of tetrafluoromethane and hexafluoroethane from primary aluminium production
- Chemical and physical properties table
- WebBook page for CF4

