Tebbe's reagent
Encyclopedia
The Tebbe reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It used in the methylenation of carbonyl
compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free technique
s.
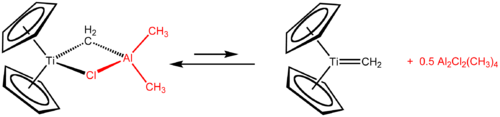
Tebbe's reagent contains two tetrahedral centers linked by a pair of bridging ligand
s. Titanium features two cyclopentadienyl
rings, and aluminium features two methyl ligands. The titanium and aluminium atoms are bridged by both CH2
and chloride ligands. This compound exhibits a nearly square (Ti-CH2-Al-Cl) bridge. The Tebbe reagent was the first reported compound where a methylene group bridges a transition metal (Ti) and a main group metal (Al).
and trimethylaluminium
in toluene
solution.
After about 3 days, the product is obtained after recrystallization to remove Al(CH3)2Cl. Although syntheses using the isolated Tebbe reagent give a cleaner product, successful procedures using the reagent "in situ" have been reported. Instead of isolating the Tebbe reagent, the solution is merely cooled in an ice bath or dry ice bath before adding the starting material.
An alternative but less convenient synthesis entails the use of dimethyltitanocene (Petasis reagent):
One drawback to this method, aside from requiring Cp2Ti(CH3)2, is the difficulty of separating product from unreacted starting reagent.
, which generates the active Schrock carbene.
Also analogous to the Wittig reagent, the reactivity appears to be driven by the high oxophilicity of Ti(IV). The Schrock carbene (1) reacts with carbonyl compounds (2) to give a postulated oxatitanacyclobutane intermediate (3). This cyclic intermediate has never been directly isolated, presumably because it breaks down immediately to the produce the desired alkene
(5).
for carbonyl methylenation. This conversion can also be effected using the Wittig reaction
, although the Tebbe reagent is more efficient especially for sterically encumbered carbonyls. Furthermore, the Tebbe reagent is less basic than the Wittig reagent and does not give the β-elimination products.
Methylenation reactions also occur for aldehydes as well as esters, lactones and amide
s. The Tebbe reagent converts esters and lactones to enol ethers and amides to enamines. In compounds containing both ketone and ester groups, the ketone selectively reacts in the presence of one equivalent of the Tebbe reagent.
The Tebbe reagent methylenates carbonyls without racemizing a chiral
α carbon. For this reason, the Tebbe reagent has found applications in reactions of sugars where maintenance of stereochemistry
can be critical.
The Tebbe reagent reacts with acid chlorides to form titanium enolates by replacing Cl-.
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free technique
Air-free technique
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen...
s.
Tebbe's reagent contains two tetrahedral centers linked by a pair of bridging ligand
Bridging ligand
A bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are...
s. Titanium features two cyclopentadienyl
Cyclopentadienyl complex
A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups . Based on the type of bonding between the metals and the cyclopentadienyl]] moieties, cyclopentadienyl complexes are classified into the following three categories: a) π-complexes, b) σ-complexes, and c) ionic...
rings, and aluminium features two methyl ligands. The titanium and aluminium atoms are bridged by both CH2
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
and chloride ligands. This compound exhibits a nearly square (Ti-CH2-Al-Cl) bridge. The Tebbe reagent was the first reported compound where a methylene group bridges a transition metal (Ti) and a main group metal (Al).
Preparation
The Tebbe reagent is synthesized from titanocene dichlorideTitanocene dichloride
Titanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
and trimethylaluminium
Trimethylaluminium
Trimethylaluminium is the chemical compound with the formula Al26, abbreviated as Al2Me6, 2 or the abbreviation TMA. This pyrophoric, colorless liquid is an industrially important organoaluminium compound...
in toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
solution.
-
- Cp2TiCl2 + 2 Al(CH3)3 → CH4 + Cp2TiCH2AlCl(CH3)2 + Al(CH3)2Cl
After about 3 days, the product is obtained after recrystallization to remove Al(CH3)2Cl. Although syntheses using the isolated Tebbe reagent give a cleaner product, successful procedures using the reagent "in situ" have been reported. Instead of isolating the Tebbe reagent, the solution is merely cooled in an ice bath or dry ice bath before adding the starting material.
An alternative but less convenient synthesis entails the use of dimethyltitanocene (Petasis reagent):
-
- Cp2Ti(CH3)2 + Al(CH3)2Cl → Cp2TiCH2AlCl(CH3)2 + CH4
One drawback to this method, aside from requiring Cp2Ti(CH3)2, is the difficulty of separating product from unreacted starting reagent.
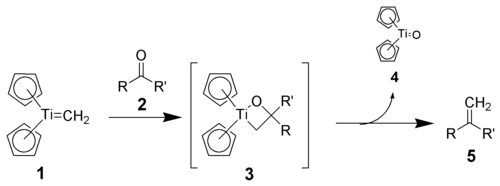
Reaction mechanism
Tebbe's reagent itself does not react with carbonyl compounds, but must first be treated with a mild Lewis base, such as pyridinePyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, which generates the active Schrock carbene.
Also analogous to the Wittig reagent, the reactivity appears to be driven by the high oxophilicity of Ti(IV). The Schrock carbene (1) reacts with carbonyl compounds (2) to give a postulated oxatitanacyclobutane intermediate (3). This cyclic intermediate has never been directly isolated, presumably because it breaks down immediately to the produce the desired alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
(5).
Scope
The Tebbe reagent is used in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
for carbonyl methylenation. This conversion can also be effected using the Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
, although the Tebbe reagent is more efficient especially for sterically encumbered carbonyls. Furthermore, the Tebbe reagent is less basic than the Wittig reagent and does not give the β-elimination products.
Methylenation reactions also occur for aldehydes as well as esters, lactones and amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s. The Tebbe reagent converts esters and lactones to enol ethers and amides to enamines. In compounds containing both ketone and ester groups, the ketone selectively reacts in the presence of one equivalent of the Tebbe reagent.
The Tebbe reagent methylenates carbonyls without racemizing a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
α carbon. For this reason, the Tebbe reagent has found applications in reactions of sugars where maintenance of stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
can be critical.
The Tebbe reagent reacts with acid chlorides to form titanium enolates by replacing Cl-.
See also
- Petasis reagentPetasis reagentThe Petasis reagent is dimethyl titanocene, Cp2TiMe2, readily prepared by the reaction of methylmagnesium chloride or methyllithium with titanocene dichloride:...
- Peterson olefinationPeterson olefinationThe Peterson olefination is the chemical reaction of α-silyl carbanions 1 with ketones to form a β-hydroxysilane 2 which eliminates to form alkenes 3.Several reviews have been published....
- Titanocene dichlorideTitanocene dichlorideTitanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
- Wittig reactionWittig reactionThe Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
- Kauffmann olefinationKauffmann olefinationThe Kauffmann olefination is a chemical reaction to convert aldehydes and ketones to methylene-olefins. This reaction was discovered by the German chemist Thomas Kauffmann and is related to the better known Tebbe olefination or Wittig reaction....