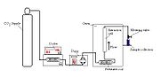
Supercritical fluid extraction
Encyclopedia
Supercritical Fluid Extraction (SFE) is the process of separating one component (the extractant) from another (the matrix) using supercritical fluid
s as the extracting solvent
. Extraction is usually from a solid
matrix, but can also be from liquid
s. SFE can be used as a sample preparation
step for analytical
purposes, or on a larger scale to either strip unwanted material from a product (e.g. decaffeination
) or collect a desired product (e.g. essential oil
s). Carbon dioxide
(CO2) is the most used supercritical fluid, sometimes modified by co-solvents such as ethanol
or methanol
. Extraction conditions for supercritical CO2 are above the critical temperature of 31°C and critical pressure of 74 bar
. Addition of modifiers may slightly alter this. The discussion below will mainly refer to extraction with CO2, except where specified.
s such as hexane
or dichloromethane
. There will always be some residual solvent left in the extract and matrix, and there is always some level of environmental contamination from their use. In contrast, carbon dioxide is easy to remove simply by reducing the pressure, leaving almost no trace, purchased CO2 has almost always been reclaimed, which reduces the total carbon foot-print. The use of SFE with CO2 is approved by the Soil Association
for organic
products. The CO2 used is largely a by product of industrial processes or brewing, and its use in SFE does not cause any extra emissions.
from a plant with low pressures (100 bar), whereas liquid extraction would also remove lipids. Lipids can be removed using pure CO2 at higher pressures, and then phospholipids can be removed by adding ethanol to the solvent.
-based process, with the solvent required to diffuse into the matrix, and the extracted material to diffuse out of the matrix into the solvent. Diffusivities are much faster in supercritical fluids than in liquids, and therefore extraction can occur faster. Also, there is no surface tension
and viscosities
are much lower than in liquids, so the solvent can penetrate into small pores within the matrix inaccessible to liquids. Both the higher diffusivity and lower viscosity significantly increase the speed of the extraction: An extraction using an organic liquid may take several hours, whereas supercritical fluid extraction can be completed in 10 to 60 minutes.
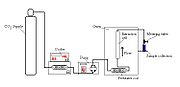
s are most common. The pump heads will usually require cooling, and the CO2 will also be cooled before entering the pump.
s can range from simple tubing to more sophisticated purpose built vessels with quick release fittings. The pressure requirement is at least 74 bar, and most extractions are conducted at under 350 bar. However, sometimes higher pressures will be needed, such as extraction of vegetable oils, where pressures of 800 bar are sometimes required for complete miscibility of the two phase
s.
The vessel must be equipped with a means of heating. It can be placed inside an oven for small vessels, or an oil or electrically heated jacket for larger vessels. Care must be taken if rubber seals are used on the vessel, as the CO2 may dissolve in the rubber, causing swelling, and the rubber will rupture on depressurization.
expansion of the CO2 results in significant cooling. This is problematic if water or other extracted material is present in the sample, as this may freeze in the restrictor or valve and cause blockages.
for collection. It is possible to fractionate the dissolved material using a series of vessels at reducing pressure. The CO2 can be recycled or depressurized to atmospheric pressure and vented. For analytical SFE, the pressure is usually dropped to atmospheric, and the now gaseous carbon dioxide bubbled through a solvent to trap the precipitated components.
must be provided to prevent excessive cooling. For small scale extractions, such as for analytical purposes, it is usually sufficient to pre-heat the fluid in a length of tubing inside the oven containing the extraction cell. The restrictor can be electrically heated, or even heated with a hairdryer. For larger systems, the energy required during each stage of the process can be calculated using the thermodynamic properties of the supercritical fluid.
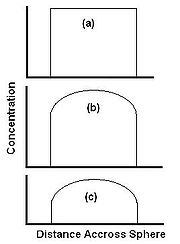 There are two essential steps to SFE, transport (by diffusion or otherwise) from with the solid particles to the surface, and dissolution in the supercritical fluid. Other factors, such as diffusion into the particle by the SF and reversible
There are two essential steps to SFE, transport (by diffusion or otherwise) from with the solid particles to the surface, and dissolution in the supercritical fluid. Other factors, such as diffusion into the particle by the SF and reversible
release such as desorption from an active site are sometimes significant, but not dealt with in detail here. Figure 2 shows the stages during extraction from a spherical particle where at the start of the extraction the level of extractant is equal across the whole sphere (Fig. 2a). As extraction commences, material is initially extracted from the edge of the sphere, and the concentration in the center is unchanged (Fig 2b). As the extraction progresses, the concentration in the center of the sphere drops as the extractant diffuses towards the edge of the sphere (Figure 2c).
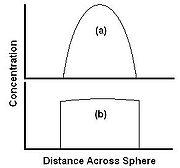 The relative rates of diffusion and dissolution are illustrated by two extreme cases in Figure 3. Figure 3a shows a case where dissolution is fast relative to diffusion. The material is carried away from the edge faster than it can diffuse from the center, so the concentration at the edge drops to zero. The material is carried away as fast as it arrives at the surface, and the extraction is completely diffusion limited. Here the rate of extraction can be increased by increasing diffusion rate, for example raising the temperature, but not by increasing the flow rate of the solvent. Figure 3b shows a case where solubility is low relative to diffusion. The extractant is able to diffuse to the edge faster than it can be carried away by the solvent, and the concentration profile is flat. In this case, the extraction rate can be increased by increasing the rate of dissolution, for example by increasing flow rate of the solvent.
The relative rates of diffusion and dissolution are illustrated by two extreme cases in Figure 3. Figure 3a shows a case where dissolution is fast relative to diffusion. The material is carried away from the edge faster than it can diffuse from the center, so the concentration at the edge drops to zero. The material is carried away as fast as it arrives at the surface, and the extraction is completely diffusion limited. Here the rate of extraction can be increased by increasing diffusion rate, for example raising the temperature, but not by increasing the flow rate of the solvent. Figure 3b shows a case where solubility is low relative to diffusion. The extractant is able to diffuse to the edge faster than it can be carried away by the solvent, and the concentration profile is flat. In this case, the extraction rate can be increased by increasing the rate of dissolution, for example by increasing flow rate of the solvent. 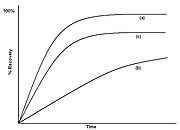 The extraction curve of % recovery against time can be used to elucidate the type of extraction occurring. Figure 4(a) shows a typical diffusion controlled curve. The extraction is initially rapid, until the concentration at the surface drops to zero, and the rate then becomes much slower. The % extracted eventually approaches 100%. Figure 4(b) shows a curve for a solubility limited extraction. The extraction rate is almost constant, and only flattens off towards the end of the extraction. Figure 4(c) shows a curve where there are significant matrix effects, where there is some sort of reversible interaction with the matrix, such as desorption from an active site. The recovery flattens off, and if the 100% value is not known, then it is hard to tell that extraction is less than complete.
The extraction curve of % recovery against time can be used to elucidate the type of extraction occurring. Figure 4(a) shows a typical diffusion controlled curve. The extraction is initially rapid, until the concentration at the surface drops to zero, and the rate then becomes much slower. The % extracted eventually approaches 100%. Figure 4(b) shows a curve for a solubility limited extraction. The extraction rate is almost constant, and only flattens off towards the end of the extraction. Figure 4(c) shows a curve where there are significant matrix effects, where there is some sort of reversible interaction with the matrix, such as desorption from an active site. The recovery flattens off, and if the 100% value is not known, then it is hard to tell that extraction is less than complete.
, then the essential factors are complete extraction in the shortest time. However, for production of an essential oil extract from a plant, then quantity of CO2 used will be a significant cost, and "complete" extraction not required, a yield of 70 - 80% perhaps being sufficient to provide economic returns. In another case, the selectivity may be more important, and a reduced rate of extraction will be preferable if it provides greater discrimination. Therefore few comments can be made which are universally applicable. However, some general principles are outlined below.
s in particular are swelled dramatically by CO2, with diffusion being increased by several orders of magnitude
in some cases.
Supercritical fluid
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
s as the extracting solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
. Extraction is usually from a solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
matrix, but can also be from liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
s. SFE can be used as a sample preparation
Sample preparation (analytical chemistry)
In analytical chemistry, sample preparation refers to the ways in which a sample is treated prior to its analysis. Preparation is a very important step in most analytical techniques, because the techniques are often not responsive to the analyte in its in-situ form, or the results are distorted by...
step for analytical
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
purposes, or on a larger scale to either strip unwanted material from a product (e.g. decaffeination
Decaffeination
Decaffeination is the act of removing caffeine from coffee beans, cocoa, tea leaves and other caffeine-containing materials. Despite removal of caffeine, many decaffeinated drinks still have around 1-2% of the...
) or collect a desired product (e.g. essential oil
Essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile aroma compounds from plants. Essential oils are also known as volatile oils, ethereal oils or aetherolea, or simply as the "oil of" the plant from which they were extracted, such as oil of clove...
s). Carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) is the most used supercritical fluid, sometimes modified by co-solvents such as ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
or methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
. Extraction conditions for supercritical CO2 are above the critical temperature of 31°C and critical pressure of 74 bar
Bar (unit)
The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
. Addition of modifiers may slightly alter this. The discussion below will mainly refer to extraction with CO2, except where specified.
Environmental improvement and reduced product contamination
SFE is an alternative to liquid extraction using solventSolvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s such as hexane
Hexane
Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
or dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
. There will always be some residual solvent left in the extract and matrix, and there is always some level of environmental contamination from their use. In contrast, carbon dioxide is easy to remove simply by reducing the pressure, leaving almost no trace, purchased CO2 has almost always been reclaimed, which reduces the total carbon foot-print. The use of SFE with CO2 is approved by the Soil Association
Soil Association
The Soil Association is a charity based in the United Kingdom. Founded in 1946, it has over 27,000 members today. Its activities include campaign work on issues including opposition to intensive farming, support for local purchasing and public education on nutrition; as well the certification of...
for organic
Organic certification
Organic certification is a certification process for producers of organic food and other organic agricultural products. In general, any business directly involved in food production can be certified, including seed suppliers, farmers, [food] processors, retailers and restaurants.Requirements vary...
products. The CO2 used is largely a by product of industrial processes or brewing, and its use in SFE does not cause any extra emissions.
Selectivity
The properties of a supercritical fluid can be altered by varying the pressure and temperature, allowing selective extraction. For example, volatile oils can be extractedExtraction (fragrance)
Fragrance extraction refers to the extraction of aromatic compounds from raw materials, using methods such as distillation, solvent extraction, expression, or enfleurage...
from a plant with low pressures (100 bar), whereas liquid extraction would also remove lipids. Lipids can be removed using pure CO2 at higher pressures, and then phospholipids can be removed by adding ethanol to the solvent.
Speed
Extraction is a diffusionDiffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
-based process, with the solvent required to diffuse into the matrix, and the extracted material to diffuse out of the matrix into the solvent. Diffusivities are much faster in supercritical fluids than in liquids, and therefore extraction can occur faster. Also, there is no surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
and viscosities
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
are much lower than in liquids, so the solvent can penetrate into small pores within the matrix inaccessible to liquids. Both the higher diffusivity and lower viscosity significantly increase the speed of the extraction: An extraction using an organic liquid may take several hours, whereas supercritical fluid extraction can be completed in 10 to 60 minutes.
Limitations
The requirement for high pressures increases the cost compared to conventional liquid extraction, so SFE will only be used where there are significant advantages. Carbon dioxide itself is non-polar, and has somewhat limited dissolving power, so cannot always be used as a solvent on its own, particularly for polar solutes. The use of modifiers increases the range of materials which can be extracted. Food grade modifiers such as ethanol can often be used, and can also help in the collection of the extracted material, but reduces some of the benefits of using a solvent which is gaseous at room temperature.Procedure
The system must contain a pump for the CO2, a pressure cell to contain the sample, a means of maintaining pressure in the system and a collecting vessel. The liquid is pumped to a heating zone, where it is heated to supercritical conditions. It then passes into the extraction vessel, where it rapidly diffuses into the solid matrix and dissolves the material to be extracted. The dissolved material is swept from the extraction cell into a separator at lower pressure, and the extracted material settles out. The CO2 can then be cooled, re-compressed and recycled, or discharged to atmosphere.
Pumps
Carbon dioxide is usually pumped as a liquid, usually below 5°C and a pressure of about 50 bar. The solvent is pumped as a liquid as it is then almost incompressible; if it was pumped as a supercritical fluid, much of the pump stroke would be "used up" in compressing the fluid, rather than pumping it. For small scale extractions (up to a few grams / minute), reciprocating pumps or syringe pumps are often used. For larger scale extractions, diaphragm pumpDiaphragm pump
A diaphragm pump is a positive displacement pump that uses a combination of the reciprocating action of a rubber, thermoplastic or teflon diaphragm and suitable non-return check valves to pump a fluid...
s are most common. The pump heads will usually require cooling, and the CO2 will also be cooled before entering the pump.
Pressure vessels
Pressure vesselPressure vessel
A pressure vessel is a closed container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.The pressure differential is dangerous and many fatal accidents have occurred in the history of their development and operation. Consequently, their design,...
s can range from simple tubing to more sophisticated purpose built vessels with quick release fittings. The pressure requirement is at least 74 bar, and most extractions are conducted at under 350 bar. However, sometimes higher pressures will be needed, such as extraction of vegetable oils, where pressures of 800 bar are sometimes required for complete miscibility of the two phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
s.
The vessel must be equipped with a means of heating. It can be placed inside an oven for small vessels, or an oil or electrically heated jacket for larger vessels. Care must be taken if rubber seals are used on the vessel, as the CO2 may dissolve in the rubber, causing swelling, and the rubber will rupture on depressurization.
Pressure maintenance
The pressure in the system must be maintained from the pump right through the pressure vessel. In smaller systems (up to about 10 mL / min) a simple restrictor can be used. This can be either a capillary tube cut to length, or a needle valve which can be adjusted to maintain pressure at different flow rates. In larger systems a back pressure regulator will be used, which maintains pressure upstream of the regulator by means of a spring, compressed air, or electronically driven valve. Whichever is used, heating must be supplied, as the adiabaticAdiabatic process
In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
expansion of the CO2 results in significant cooling. This is problematic if water or other extracted material is present in the sample, as this may freeze in the restrictor or valve and cause blockages.
Collection
The supercritical solvent is passed into a vessel at lower pressure than the extraction vessel. The density, and hence dissolving power, of supercritical fluids varies sharply with pressure, and hence the solubility in the lower density CO2 is much lower, and the material precipitatesPrecipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
for collection. It is possible to fractionate the dissolved material using a series of vessels at reducing pressure. The CO2 can be recycled or depressurized to atmospheric pressure and vented. For analytical SFE, the pressure is usually dropped to atmospheric, and the now gaseous carbon dioxide bubbled through a solvent to trap the precipitated components.
Heating and cooling
This is an important aspect. The fluid is cooled before pumping to maintain liquid conditions, then heated after pressurization. As the fluid is expanded into the separator, heatHeat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
must be provided to prevent excessive cooling. For small scale extractions, such as for analytical purposes, it is usually sufficient to pre-heat the fluid in a length of tubing inside the oven containing the extraction cell. The restrictor can be electrically heated, or even heated with a hairdryer. For larger systems, the energy required during each stage of the process can be calculated using the thermodynamic properties of the supercritical fluid.
Simple model of SFE

Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
release such as desorption from an active site are sometimes significant, but not dealt with in detail here. Figure 2 shows the stages during extraction from a spherical particle where at the start of the extraction the level of extractant is equal across the whole sphere (Fig. 2a). As extraction commences, material is initially extracted from the edge of the sphere, and the concentration in the center is unchanged (Fig 2b). As the extraction progresses, the concentration in the center of the sphere drops as the extractant diffuses towards the edge of the sphere (Figure 2c).


Optimization
The optimum will depend on the purpose of the extraction. For an analytical extraction to determine, say, antioxidant content of a polymerPolymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
, then the essential factors are complete extraction in the shortest time. However, for production of an essential oil extract from a plant, then quantity of CO2 used will be a significant cost, and "complete" extraction not required, a yield of 70 - 80% perhaps being sufficient to provide economic returns. In another case, the selectivity may be more important, and a reduced rate of extraction will be preferable if it provides greater discrimination. Therefore few comments can be made which are universally applicable. However, some general principles are outlined below.
Maximizing diffusion
This can be achieved by increasing the temperature, swelling the matrix, or reducing the particle size. Matrix swelling can sometimes be increased by increasing the pressure of the solvent, and by adding modifiers to the solvent. Some polymers and elastomerElastomer
An elastomer is a polymer with the property of viscoelasticity , generally having notably low Young's modulus and high yield strain compared with other materials. The term, which is derived from elastic polymer, is often used interchangeably with the term rubber, although the latter is preferred...
s in particular are swelled dramatically by CO2, with diffusion being increased by several orders of magnitude
Order of magnitude
An order of magnitude is the class of scale or magnitude of any amount, where each class contains values of a fixed ratio to the class preceding it. In its most common usage, the amount being scaled is 10 and the scale is the exponent being applied to this amount...
in some cases.

