
Supercritical drying
Encyclopedia
Microelectromechanical systems
Microelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
(MEMS), the drying of spice
Spice
A spice is a dried seed, fruit, root, bark, or vegetative substance used in nutritionally insignificant quantities as a food additive for flavor, color, or as a preservative that kills harmful bacteria or prevents their growth. It may be used to flavour a dish or to hide other flavours...
s, the production of aerogel
Aerogel
Aerogel is a synthetic porous material derived from a gel, in which the liquid component of the gel has been replaced with a gas. The result is a solid with extremely low density and thermal conductivity...
, and in the preparation of biological specimens for scanning electron microscopy
Scanning electron microscope
A scanning electron microscope is a type of electron microscope that images a sample by scanning it with a high-energy beam of electrons in a raster scan pattern...
.
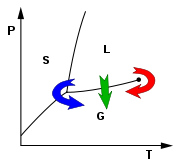
As the substance in a liquid body crosses the boundary from liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
to gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
(see green arrow in phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
), the liquid changes into gas at a finite rate, while the liquid body's volume decreases. When this happens within a heterogeneous environment, surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
in the liquid body pulls against any solid structures the liquid might be in contact with. Delicate structures such as cell wall
Cell wall
The cell wall is the tough, usually flexible but sometimes fairly rigid layer that surrounds some types of cells. It is located outside the cell membrane and provides these cells with structural support and protection, and also acts as a filtering mechanism. A major function of the cell wall is to...
s, the dendrites in silica gel
Silica gel
Silica gel is a granular, vitreous, porous form of silica made synthetically from sodium silicate. Despite its name, silica gel is a solid. It is a naturally occurring mineral that is purified and processed into either granular or beaded form...
, and the tiny machinery of microelectromechanical devices, tend to be broken apart by this surface tension as the liquid–solid interface moves by.
To avoid this, the sample can be brought via two possible alternate paths from the liquid phase to the gas phase without crossing the liquid–gas boundary on the phase diagram. In freeze-drying, this means going around to the left (low temperature, low pressure; blue arrow). However, some structures are disrupted even by the solid–gas boundary. Supercritical drying, on the other hand, goes around the line to the right, on the high-temperature, high-pressure side (red arrow). This route from liquid to gas does not cross any phase boundary, instead passing through the supercritical region, where the distinction between gas and liquid ceases to apply. Densities of the liquid phase and vapor phase becomes equal at critical point of drying.
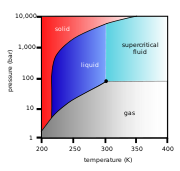
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(critical point 304.25 K at 7.39 MPa
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
or 31.1 °C
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
at 1072 psi) and freon (≈300 K at 3.5–4 MPa or 25–0 °C at 500–600 psi). Nitrous oxide
Nitrous oxide
Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
has similar physical behavior to carbon dioxide, but is a powerful oxidizer
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
in its supercritical state. Supercritical water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
is also a powerful oxidizer, partly because its critical point occurs at such a high temperature (647 K, 374 °C) and pressure (22.064 MPa, 3212 psi).
In most such processes, acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
is first used to wash away all water, exploiting the complete miscibility
Miscibility
Miscibility is the property of liquids to mix in all proportions, forming a homogeneous solution. In principle, the term applies also to other phases , but the main focus is usually on the solubility of one liquid in another...
of these two fluids. The acetone is then washed away with high pressure liquid carbon dioxide, the industry standard now that freon is unavailable. The liquid carbon dioxide is then heated until its temperature goes beyond the critical point, at which time the pressure can be gradually released, allowing the gas to escape and leaving a dried product.

