
Sulfite oxidase
Encyclopedia
Sulfite oxidase is an enzyme
in the mitochondria of all eukaryote
s. It oxidizes sulfite
to sulfate
and, via cytochrome c
, transfers the electrons produced to the electron transport chain
, allowing generation of ATP
in oxidative phosphorylation
. This is the last step in the metabolism of sulfur
-containing compounds and the sulfate is excreted.
Sulfite oxidase is a metallo-enzyme that utilizes a molybdopterin
cofactor and a heme
group. It is one of the cytochromes b5
and belongs to the enzyme super-family of molybdenum oxotransferase
s that also includes DMSO reductase
, xanthine oxidase
, and nitrite reductase
.
In mammals, the expression levels of sulfite oxidase is high in the liver, kidney, and heart, and very low in spleen, brain, skeletal muscle, and blood.
s forming a loop. The N-terminal domain has a heme
cofactor with three adjacent antiparallel beta sheet
s and five alpha helices
. The C-terminal domain hosts a molybdopterin cofactor that is surrounded by thirteen beta sheets and three alpha helices. The molybdopterin
cofactor has a Mo(VI) center, which is bonded to a sulfur from cysteine
, an ene-dithiolate from pyranopterin, and two terminal oxygens. It is at this molybdenum center that the catalytic oxidation of sulfite takes place.
The active site of sulfite oxidase contains the molybdopterin
cofactor and supports molybdenum in its highest oxidation state, +6 (MoVI). In the enzyme's oxidized state, molybdenum is coordinated by a cysteine thiolate, the dithiolene
group of molybdopterin, and two terminal oxygen atoms (oxo
s). Upon reacting with sulfite, one oxygen atom is transferred to sulfite to produce sulfate, and the molybdenum center is reduced by two electrons to MoIV. Water then displaces sulfate, and the removal of two protons (H+) and two electrons (e-) returns the active site to its original state. A key feature of this oxygen atom transfer enzyme is that the oxygen atom being transferred arises from water, not from dioxygen (O2).
in foods. Lack of functional sulfite oxidase causes a disease known as sulfite oxidase deficiency. This rare but fatal disease causes neurological disorders, mental retardation, physical deformities, the degradation of the brain, and death. Reasons for the lack of functional sulfite oxidase include a genetic
defect that leads to the absence of a molybdopterin
cofactor
and point mutation
s in the enzyme. A G473D mutation impairs dimerization and catalysis in human sulfite oxidase.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
in the mitochondria of all eukaryote
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
s. It oxidizes sulfite
Sulfite
Sulfites are compounds that contain the sulfite ion SO. The sulfite ion is the conjugate base of bisulfite. Although the acid itself is elusive, its salts are widely used.-Structure:...
to sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
and, via cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
, transfers the electrons produced to the electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
, allowing generation of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
in oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
. This is the last step in the metabolism of sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
-containing compounds and the sulfate is excreted.
Sulfite oxidase is a metallo-enzyme that utilizes a molybdopterin
Molybdopterin
Molybdopterins, when reacted with molybdenum or tungsten in the form of molybdate or tungstate, are a class of cofactors found in most molybdenum and all tungsten enzymes...
cofactor and a heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group. It is one of the cytochromes b5
Cytochrome b5
Cytochromes b5 are ubiquitous electron transport hemoproteins found in animals, plants, fungi and purple phototrophic bacteria. The microsomal and mitochondrial variants are membrane-bound, while bacterial and those from erythrocytes and other animal tissues are water-soluble...
and belongs to the enzyme super-family of molybdenum oxotransferase
Molybdenum oxotransferase
The enzyme super-family of molybdenum oxotransferases all contain molybdenum, and promote oxygen atom transfer reactions.Enzymes in this family include DMSO reductase, xanthine oxidase, nitrite reductase, and sulfite oxidase....
s that also includes DMSO reductase
DMSO reductase
DMSO reductase is a molybdenum-containing enzyme capable of reducing dimethyl sulfoxide to dimethyl sulfide . This enzyme serves as the terminal reductase under anaerobic conditions in some bacteria, with DMSO being the terminal electron acceptor...
, xanthine oxidase
Xanthine oxidase
Xanthine oxidase Xanthine oxidase Xanthine oxidase (XO (sometimes 'XAO'), a form of xanthine oxidoreductase that generates reactive oxygen species. Is an enzyme that catalyzes the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid...
, and nitrite reductase
Nitrite reductase
Nitrite reductase refers to any of several classes of enzymes that catalyze the reduction of nitrite. There are two classes of NIR's. A multi haem enzyme reduces NO2 to a variety of products. Copper containing enzymes carry out a single electron transfer to produce nitric oxide.- Iron based...
.
In mammals, the expression levels of sulfite oxidase is high in the liver, kidney, and heart, and very low in spleen, brain, skeletal muscle, and blood.
Structure
As a homodimer, sulfite oxidase contains two identical subunits with an N-terminal domain and a C-terminal domain. These two domains are connected by ten amino acidAmino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s forming a loop. The N-terminal domain has a heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
cofactor with three adjacent antiparallel beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
s and five alpha helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
. The C-terminal domain hosts a molybdopterin cofactor that is surrounded by thirteen beta sheets and three alpha helices. The molybdopterin
Molybdopterin
Molybdopterins, when reacted with molybdenum or tungsten in the form of molybdate or tungstate, are a class of cofactors found in most molybdenum and all tungsten enzymes...
cofactor has a Mo(VI) center, which is bonded to a sulfur from cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
, an ene-dithiolate from pyranopterin, and two terminal oxygens. It is at this molybdenum center that the catalytic oxidation of sulfite takes place.
Active site and mechanism
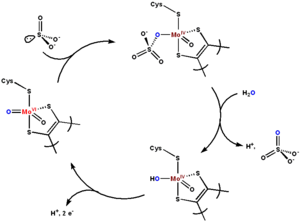 |
The active site of sulfite oxidase contains the molybdopterin
Molybdopterin
Molybdopterins, when reacted with molybdenum or tungsten in the form of molybdate or tungstate, are a class of cofactors found in most molybdenum and all tungsten enzymes...
cofactor and supports molybdenum in its highest oxidation state, +6 (MoVI). In the enzyme's oxidized state, molybdenum is coordinated by a cysteine thiolate, the dithiolene
Dithiolene
Metal dithiolene complexes are complexes containing dithiolene ligands. Dithiolene ligands are unsaturated bidentate ligand wherein the two donor atoms are sulfur. Dithiolenes are often referred to as "metallodithiolenes" or "dithiolene complexes"...
group of molybdopterin, and two terminal oxygen atoms (oxo
Oxo
OXO was the first digital graphical computer game, a version of Tic-tac-toe.It is also the first puzzler game; As seen on Ginuess World Records 2010 Gamer's Edition.OXO Was first released in 1951, That makes it one of the oldest games standing....
s). Upon reacting with sulfite, one oxygen atom is transferred to sulfite to produce sulfate, and the molybdenum center is reduced by two electrons to MoIV. Water then displaces sulfate, and the removal of two protons (H+) and two electrons (e-) returns the active site to its original state. A key feature of this oxygen atom transfer enzyme is that the oxygen atom being transferred arises from water, not from dioxygen (O2).
Deficiency
Sulfite oxidase is required to metabolize the sulfur-containing amino acids cysteine and methionineMethionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
in foods. Lack of functional sulfite oxidase causes a disease known as sulfite oxidase deficiency. This rare but fatal disease causes neurological disorders, mental retardation, physical deformities, the degradation of the brain, and death. Reasons for the lack of functional sulfite oxidase include a genetic
Genetics
Genetics , a discipline of biology, is the science of genes, heredity, and variation in living organisms....
defect that leads to the absence of a molybdopterin
Molybdopterin
Molybdopterins, when reacted with molybdenum or tungsten in the form of molybdate or tungstate, are a class of cofactors found in most molybdenum and all tungsten enzymes...
cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
and point mutation
Point mutation
A point mutation, or single base substitution, is a type of mutation that causes the replacement of a single base nucleotide with another nucleotide of the genetic material, DNA or RNA. Often the term point mutation also includes insertions or deletions of a single base pair...
s in the enzyme. A G473D mutation impairs dimerization and catalysis in human sulfite oxidase.
Further reading
- Kisker, C. “Sulfite oxidase”, Messerschimdt, A.; Huber, R.; Poulos, T.; Wieghardt, K.; eds. Handbook of Metalloproteins, vol 2; John Wiley and Sons, Ltd: New York, 2002

