Suboxide
Encyclopedia
Suboxides are a class of oxide
s wherein the electropositive element is in excess relative to the “normal” oxide
s. When the electropositive element is a metal, the compounds are sometimes referred to as “metal-rich”. Thus the normal oxide of caesium
is Cs2O, which is described as a Cs+ salt of O2−. A suboxide of caesium is Cs11O3, where the charge on Cs is clearly less than 1+, but the oxide is still described as O2−. Suboxides typically feature extensive bonding between the electropositive element, often leading to clusters.
Examples of suboxides include
:
Several suboxides of caesium
and rubidium have been characterized by X-ray crystallography
. As of 1997, the inventory includes the following Rb9O2, Rb6O, Cs11O3, Cs4O, Cs7O, Cs11O3Rb, Cs11O3Rb2, and Cs11O3Rb3.
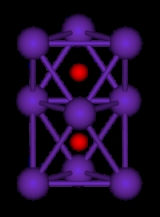
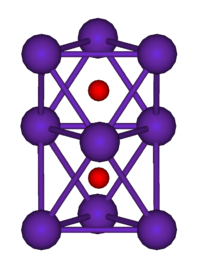
They are generally colored compounds indicating a degree of electron delocalisation. Cs7O, has a unit cell containing a Cs10O3 cluster and 10 Cs atoms. The cluster can be visualised as being composed of three face-sharing octahedra. In the picture below the caesium atoms are purple and the oxygen atoms are red. The Cs-Cs distance in the cluster is 376 pm, which is less than the Cs-Cs distance in the metal of 576 pm. Rb9O2 and Rb6O both contain the Rb9O2 cluster, which can be visualised as two face-sharing octahedra. Rb6O can be formulated as (Rb9O2)Rb3. The Rb-Rb distance in the cluster is 352 pm which is shorter than the Rb-Rb in the metal of 485 pm. It is suggested that caesium suboxides play a role in the Ag-O-Cs (S1) and multialkali Na-K-Sb-Cs photocathode
s.
s (e.g. ketene
), C3O2 obeys the octet rule
.
-centered octahedral cluster
of six barium
atoms embedded in a matrix of sodium.
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s wherein the electropositive element is in excess relative to the “normal” oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s. When the electropositive element is a metal, the compounds are sometimes referred to as “metal-rich”. Thus the normal oxide of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
is Cs2O, which is described as a Cs+ salt of O2−. A suboxide of caesium is Cs11O3, where the charge on Cs is clearly less than 1+, but the oxide is still described as O2−. Suboxides typically feature extensive bonding between the electropositive element, often leading to clusters.
Examples of suboxides include
- Carbon suboxideCarbon suboxideCarbon suboxide, or tricarbon dioxide, is an oxide of carbon with chemical formula C3O2 or O=C=C=C=O. Its four cumulative double bonds make it a cumulene...
, C3O2; - Boron suboxideBoron suboxideBoron suboxide is a solid compound of boron and oxygen.Its structure is built of eight icosahedra at the apexes of the rhombohedral unit cell . Each icosahedron is composed of twelve boron atoms. Two oxygen atoms are located in the interstices along the [111] rhombohedral direction...
, B6O; - RubidiumRubidiumRubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
suboxide, Rb9O2; - Silicon suboxide, SiOx (x < 2)
Metal-containing suboxides
Suboxides are intermediates along the pathway that forms the normal oxide. Suboxides are sometimes visible when certain metals are exposed to small amounts of O2Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
:
- 22 Cs + 3 O2 → 2 Cs11O3
- 4 Cs11O3 + 5 O2 → 22 Cs2O
Several suboxides of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
and rubidium have been characterized by X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
. As of 1997, the inventory includes the following Rb9O2, Rb6O, Cs11O3, Cs4O, Cs7O, Cs11O3Rb, Cs11O3Rb2, and Cs11O3Rb3.
They are generally colored compounds indicating a degree of electron delocalisation. Cs7O, has a unit cell containing a Cs10O3 cluster and 10 Cs atoms. The cluster can be visualised as being composed of three face-sharing octahedra. In the picture below the caesium atoms are purple and the oxygen atoms are red. The Cs-Cs distance in the cluster is 376 pm, which is less than the Cs-Cs distance in the metal of 576 pm. Rb9O2 and Rb6O both contain the Rb9O2 cluster, which can be visualised as two face-sharing octahedra. Rb6O can be formulated as (Rb9O2)Rb3. The Rb-Rb distance in the cluster is 352 pm which is shorter than the Rb-Rb in the metal of 485 pm. It is suggested that caesium suboxides play a role in the Ag-O-Cs (S1) and multialkali Na-K-Sb-Cs photocathode
Photocathode
A photocathode is a negatively charged electrode in a light detection device such as a photomultiplier or phototube that is coated with a photosensitive compound...
s.
 |
 |
| Rb9O2 cluster | Cs11O3 cluster |
Carbon suboxide
The suboxide of carbon adopts an unremarkable structure. As for related organic cumuleneCumulene
A cumulene is a chemical compound with two or more cumulative double bonds, for example butatriene , H2C=C=C=CH2. Unlike alkanes and most alkenes, cumulenes tend to be rigid, which makes them appealing for molecular nanotechnology. Polyynes are another kind of rigid carbon chains...
s (e.g. ketene
Ketene
A ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
), C3O2 obeys the octet rule
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
.
Related compounds
Subnitrides are also known, e.g. Na16Ba6N, which features an nitrideNitride
In chemistry, a nitride is a compound of nitrogen where nitrogen has a formal oxidation state of −3. Nitrides are a large class of compounds with a wide range of properties and applications....
-centered octahedral cluster
Cluster chemistry
In chemistry, a cluster is an ensemble of bound atoms intermediate in size between a molecule and a bulk solid. Clusters exist of diverse stoichiometries and nuclearities. For example, carbon and boron atoms form fullerene and borane clusters, respectively. Transition metals and main group...
of six barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
atoms embedded in a matrix of sodium.

