
Strychnine total synthesis
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
describes the total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of the complex biomolecule
Biomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
strychnine
Strychnine
Strychnine is a highly toxic , colorless crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine causes muscular convulsions and eventually death through asphyxia or sheer exhaustion...
. The first reported method by the group of Robert Burns Woodward
Robert Burns Woodward
Robert Burns Woodward was an American organic chemist, considered by many to be the preeminent organic chemist of the twentieth century...
in 1954 is considered a classic in this research field.
At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii
Strychnos ignatia
Strychnos ignatia is a tree in the Loganiaceae family, native to the Philippines and parts of China. It is named for Saint Ignatius of Loyola, the founder of the Jesuit missionary order.-Fruit:...
by Pierre Joseph Pelletier
Pierre Joseph Pelletier
Pierre-Joseph Pelletier was a French chemist who did notable research on vegetable alkaloids, and was the co-discoverer of quinine and strychnine.- Further reading :...
and Joseph Bienaimé Caventou
Joseph Bienaimé Caventou
Joseph Bienaimé Caventou was a French chemist.He was a professor at the École de Pharmacie in Paris. He collaborated with Pierre-Joseph Pelletier in a Parisian laboratory located behind an apothecary. He was a pioneer in the use of mild solvents to isolate a number of active ingredients from...
in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs
Hermann Leuchs
-Life:Leuchs studied chemistry at the University of Munich from 1898 till he changed to the University of Berlin He earned his Ph.D. with Hermann Emil Fischer in 1902 in Berlin. He steadily advanced in the hierarchy of the university, 1910 lecturer, assistant professor in 1914 and full professor in...
with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
in 1947 for his work on alkaloids, strychnine included.
The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
establishing the absolute configuration
Absolute configuration
An absolute configuration in stereochemistry is the spatial arrangement of the atoms of a chiral molecular entity and its stereochemical description e.g. R or S....
became available between 1947 and 1951 with publications from J. M. Bijvoet
Johannes Martin Bijvoet
Johannes Martin Bijvoet was a Dutch chemist and crystallographer at the van 't Hoff Laboratory at the University of Utrecht...
and J.H. Robertson
.
Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.
Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori,Shibasaki, Li, Fukuyama Vanderwal. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.
In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.
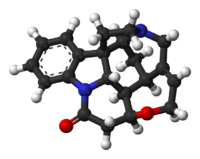
The molecule
The C21H22N2O2 strychnine molecule contains 7 rings including an indoline
Indoline
Indoline is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound is based on the indole structure, but the 2-3 bond is saturated. by oxidation/dehydrogenation it can be...
system. It has a tertiary amine group, an amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
, an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and an ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
group. The naturally occurring compound is also chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
with 6 asymmetric carbon
Asymmetric carbon
An asymmetric carbon atom is a carbon atom that is attached to four different types of atom or four different groups of atoms. Knowing the number of asymmetric carbon atoms, one can calculate the maximum possible number of stereoisomers for any given molecule as follows:As an example, malic acid...
atoms including one quaternary one.
Ring II, V synthesis
The synthesis of ring II was accomplished with an Fischer indole synthesisFischer indole synthesis
The Fischer indole synthesis isa chemical reaction that produces the aromatic heterocycle indole from a phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Hermann Emil Fischer. Today antimigraine drugs of the triptan class are often...
using phenylhydrazine
Phenylhydrazine
Phenylhydrazine is the chemical compound with the formula C6H5NHNH2. Organic chemists abbreviate the compound as PhNHNH2.- Chemical properties :...
1 and acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
derivative acetoveratrone 2 (catalyst polyphosphoric acid) to give the 2-veratrylindole 3. The veratryl group not only blocks the 2-position for further electrophilic substitution
Electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a group in a compound, typically but not always hydrogen. Electrophilic aromatic substitution is characteristic of aromatic compounds and is an important way of introducing functional groups onto benzene...
but will also become part of the strychnine skeleton. A Mannich reaction
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
with formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
and dimethylamine
Dimethylamine
Dimethylamine is an organic compound with the formula 2NH. This secondary amine is a colorless, flammable liquified gas with an ammonia-like odor. Dimethylamine is generally encountered as a solution in water at concentrations up to around 40%...
) produced gramine
Gramine
Gramine is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.-Occurrence:...
4. Alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
with iodomethane
Iodomethane
Methyl iodide, also called iodomethane, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally...
gave an intermediate quaternary ammonium salt which reacted with sodium cyanide
Sodium cyanide
Sodium cyanide is an inorganic compound with the formula NaCN. This highly toxic colorless salt is used mainly in gold mining but has other niche applications...
in a nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
to cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
5 and then in a reduction with lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
to tryptamine
Tryptamine
Tryptamine is a monoamine alkaloid found in plants, fungi, and animals. It is based around the indole ring structure, and is chemically related to the amino acid tryptophan, from which its name is derived...
6. Amine-carbonyl condensation with ethyl glyoxylate give the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
7. The reaction of this imine with TsCl
4-Toluenesulfonyl chloride
4-Toluenesulfonyl chloride is an organic compound with the formula CH3C6H4SO2Cl. This colourless, malodorous solid is a reagent widely used in organic synthesis...
in pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
to the ring-closed N-tosyl compound 8 was described by Woodward as a concerted nucleophilic enamine
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
attack and formally a Pictet–Spengler reaction. This compound should form as a diastereomeric pair but only one compound was found although which one was not investigated. Finally the newly formed double bond was reduced by sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
to indoline
Indoline
Indoline is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound is based on the indole structure, but the 2-3 bond is saturated. by oxidation/dehydrogenation it can be...
9 with the C8 hydrogen atom approaching from the least hindered side (this proton is destroyed later on in the sequence and of no importance).
Ring III, IV synthesis
Indoline 9 was acetylatedAcetylation
Acetylation describes a reaction that introduces an acetyl functional group into a chemical compound...
to N-acetyl compound 10 (acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
) and then the veratryl group was then ring-opened with ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
in aquaeous acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
to muconic ester
Muconic acid
Muconic acid is a dicarboxylic acid. There are three isomeric forms designated trans,trans-muconic acid, cis,trans-muconic acid, and cis,cis-muconic acid which differ by the geometry around the double bonds....
11 (made possible by the two electron-donating methoxide groups). This is an example of bioinspired synthesis already proposed by Woodward in 1948. Cleavage of the acetyl group and ester hydrolysis with HCl
HCL
HCL or HCl can stand for:* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia* Hardware compatibility list...
in methanol resulted in formation of pyridone ester 12 with additional isomerization of the exocyclic double bond to an endocyclic double bond (destroying one asymmetric center). Subsequent treatment with hydrogen iodide
Hydrogen iodide
Hydrogen iodide is a diatomic molecule. Aqueous solutions of HI are known as iohydroic acid or hydroiodic acid, a strong acid. Gas and aqueous solution are interconvertible...
and red phosphorus removed the tosyl group and hydrolysed both remaining ester groups to form diacid 13. Acetylation and esterfication (diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
) produced acetyl diester 14 which was then subjected to a Dieckman condensation with sodium methoxide
Sodium methoxide
Sodium methoxide is a chemical compound, with formula CH3ONa. This colourless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory...
in methanol to enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
15.
Ring VII synthesis
In order to remove the C15 alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
group, Enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
15 was converted to tosylate 16 (TsCl, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
) and then to mercaptoester 17 (sodium benzylmercaptide) which was then reduced to unsaturated ester 18 by Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. Further reduction with hydrogen / palladium on carbon
Palladium on carbon
Palladium on carbon, often referred to as Pd/C, is a form of palladium used for catalysis. It is usually used for catalytic hydrogenations in organic chemistry...
afforded the saturated ester 19. Alkaline ester hydrolysis to carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
20 was accompanied by epimerization at C14.
This particular compound was already known from strychnine degration studies. Until now all intermediates were racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
but chirality was introduced at this particular stage via chiral resolution
Chiral resolution
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs...
using quinidine
Quinidine
Quinidine is a pharmaceutical agent that acts as a class I antiarrhythmic agent in the heart. It is a stereoisomer of quinine, originally derived from the bark of the cinchona tree.-Mechanism:...
.
The C20 carbon atom was then introduced by acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
to form enol acetate 21 and the free aminoketone 22 was obtained by hydrolysis with hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
. Ring VII in intermediate 23 was closed by selenium dioxide
Selenium dioxide
Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.-Properties:...
oxidation, a process accompanied by epimerization again at C14.
The formation of 21 can be envisioned as a sequence of acylation, deprotonation, rearrangement with loss of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and again acylation:
Ring VI synthesis
To diketoneDiketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
23 was added sodium acetylide (bringing in carbon atoms 22 and 23) to give alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
24. This compound was reduced to the allyl alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
25 using the Lindlar catalyst
Lindlar catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate and treated with various forms of lead. The lead additive serves to deactivate the palladium sites. A variety of "catalyst poisons" have been used including lead acetate and lead oxide. The...
and lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
removed the remaining ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
group in 26. An allylic rearrangement
Allylic rearrangement
An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution....
to alcohol 27 (isostrychnine) was brought about by hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
in acetic acid followed by hydrolysis with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
. In the final step to (-)-strychnine 28 treatment of 27 with ethanolic potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
caused rearrangement of the C12-13 double bond and ring closure in a conjugate addition by the hydroxyl anion.
Magnus synthesis
In this effort one of strychnine's many degradation products was synthesised first (the relay compound), a compound also available in several steps from another degradation product called the Wieland-Gumlich aldehydeWieland-Gumlich aldehyde
The Wieland-Gumlich aldehyde is an indoline derived from strychnine. This compound is of some commercial interest as a chemical intermediate. It was first synthesized in 1932 by Wieland and Gumlich in 4 steps from strychnine...
. In the final leg strychnine itself was synthesised from the relay compound.
Overman synthesis
The OvermanLarry E. Overman
Larry E. Overman is Distinguished Professor of Chemistry at the University of California, Irvine. He was born in Chicago in 1943. Overman obtained a B.A. degree from Earlham College in 1965. and he completed his Ph.D. in chemistry from the University of Wisconsin–Madison in 1969, under Howard...
synthesis (1993) took a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
cyclopentene
Cyclopentene
Cyclopentene is a chemical compound with the formula 58. It is a colorless liquid with a petrol-like odor. It is one of the cycloalkenes.Cyclopentene is produced industrially in large amounts...
compound as starting material obtained by enzymatic hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of cis-1,4-diacetoxycyclopent-2-ene. This starting material was converted in several steps to trialkylstannane
Organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849...
1 which was then coupled with an aryl iodide 2 in a Stille reaction
Stille reaction
The Stille reaction is a chemical reaction coupling an organotin compound with an sp2-hybridized organic halide catalyzed by palladium. The reaction is widely used in organic synthesis....
in presence of carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
(tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0)
Trisdipalladium or Pd23 is an organometallic complex based on palladium and dibenzylideneacetone used in organic chemistry. It was discovered in 1970.-Preparation and structure:...
, triphenylarsine
Triphenylarsine
Triphenylarsine is the chemical compound with the formula As3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis...
). The internal double in 3 was converted to an epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
using tert-Butyl hydroperoxide
Tert-Butyl hydroperoxide
tert-Butyl hydroperoxide is an organic peroxide widely used in a variety of oxidation processes, for example Sharpless epoxidation...
, the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group was then converted to an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
in a Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
using Ph3P=CH2 and the TIPS
Tips
Tips may refer to:* Tip , extra payment left by guests* Tips Industries, an Indian film production company* Gambling suggestions made by a TipsterTIPS as an acronym may refer to:* Operation TIPS, Terrorism Information and Prevention System...
group was hydrolyzed (TBAF) and replaced by a trifluoroacetamide group (NH2COCF3, NaH
Nah
Nah, nah, or NaH may refer to:*Nah, Iran, a city in South Khorasan Province, Iran* Sodium hydride, whose chemical formula is NaH* The language code nah, which stands for Nahuatl...
) in 4. Cyclization (NaH) took place next, opening the epoxide ring and the trifluoroacetyl group was removed using KOH affording azabicyclooctane 5.
The key step was an aza-Cope
Cope reaction
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of an amine oxide to form an alkene and a hydroxylamine. The reaction mechanism involves an intramolcular 5-membered cyclic transition state, leading to a syn elimination product...
Mannich reaction
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
initiated by a amine-carbonyl condensation using formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
and forming 6 in a quantitative yield:
In the final sequence strychnine was obtained through the Wieland-Gumlich aldehyde
Wieland-Gumlich aldehyde
The Wieland-Gumlich aldehyde is an indoline derived from strychnine. This compound is of some commercial interest as a chemical intermediate. It was first synthesized in 1932 by Wieland and Gumlich in 4 steps from strychnine...
(10):
Intermediate 6 was acylated using methyl cyanoformate
Methyl cyanoformate
Methyl cyanoformate is the organic compound with the formula CH3OCCN. This colorless liquid is used as a reagent in organic synthesis as a source of the methoxycarbonyl group.It was also the active ingredient in the pesticide product Zyklon A....
and two protective groups (tert-butyl and ) were removed using HCl
HCL
HCL or HCl can stand for:* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia* Hardware compatibility list...
/ MeOH in 7. The C8C13 double bond was reduced with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
(MeOH/H+) to saturated ester 8 (mixture). Epimerization at C13 with sodium methoxide
Sodium methoxide
Sodium methoxide is a chemical compound, with formula CH3ONa. This colourless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory...
in MeOH produced beta-ester 9 which was reduced with diisobutylaluminium hydride
to Wieland-Gumlich aldehyde 10. Conversion of this compound with malonic acid
Malonic acid
Malonic acid is a dicarboxylic acid with structure CH22. The ionised form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's ethyl ester...
to (-)-strychnine 11 was already known as a procedure.
Kuehne synthesis
The 1993 Keuhne synthesis concerns racemicRacemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
strychnine. Starting compounds tryptamine
Tryptamine
Tryptamine is a monoamine alkaloid found in plants, fungi, and animals. It is based around the indole ring structure, and is chemically related to the amino acid tryptophan, from which its name is derived...
1 and 4,4-dimethoxy acrolein
Acrolein
Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity...
2 were reacted together with boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
to acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
3 as a single diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
in a amine-carbonyl condensation / sigmatropic rearrangement sequence.
Hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
with perchloric acid
Perchloric acid
Perchloric acid is the inorganic compound with the formula HClO4. Usually encountered as an aqueous solution, this colourless compound is a strong acid comparable in strength to sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to appr. 70% are remarkably inert,...
afforded aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
4. A Johnson-Corey-Chaykovsky reaction
Johnson-Corey-Chaykovsky reaction
The Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E.J. Corey and Michael Chaykovsky...
(trimethylsulfonium iodide / n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
) converted the aldehyde into an epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
which reacted in situ with the tertiary amine to ammonium salt 5 (contaminated with other cyclization products). Reduction (palladium on carbon
Palladium on carbon
Palladium on carbon, often referred to as Pd/C, is a form of palladium used for catalysis. It is usually used for catalytic hydrogenations in organic chemistry...
/hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
) removed the benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
group to alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
6, more reduction (sodium cyanoborohydride
Sodium cyanoborohydride
Sodium cyanoborohydride is the inorganic compound with the formula NaBH3. This colourless salt is widely used in organic synthesis for the reduction of imines.-Preparation and use:...
) and acylation
Acylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
(acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
/ pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
) produced 7 as a mixture of epimers (at C17). Ring closure of ring III to 8 was then accomplished with an aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
using lithium bis(trimethylsilyl)amide
Lithium bis(trimethylsilyl)amide
Lithium bisamide is the organosilicon compound with the formula [3Si]2NLi. This colourless solid is a strong non-nucleophilic base used for deprotonation reactions and as a ligand...
(using only the epimer with correct configuration). Even more reduction (sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
) and acylation resulted in epimeric di-acetate 9.
A DBU mediated elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
formed olefinic alcohol 10 and subsequent Swern oxidation
Swern oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide and an organic base, such as triethylamine...
have an unstable amino ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
11. In the final steps a Horner–Wadsworth–Emmons reaction (methyl 2-(diethy1phosphono)acetate) give acrylate
Acrylate
The acrylate ion is the ion of acrylic acid.Acrylates are the salts and esters of acrylic acid. They are also known as propenoates ....
ester 12 as a mixture of cis and trans isomers which could be coached into the right (trans) direction by application of light in a photochemical rearrangement, the ester group was reduced (DIBAL / boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
) to isostrychnine 13 and racemic strychnine 14 was formed by base-catalyzed ring closure as in the Woodward synthesis.
In the 1998 Keuhne synthesis of chiral (-)-strychnine the starting material was derived from chiral tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
.
Rawal synthesis
In the Rawal synthesis (1994, racemic) amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
1 and enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
2 were combined in a amine-carbonyl condensation followed by methyl chloroformate
Chloroformate
Chloroformates are a class of chemical compounds which are esters of chloroformic acid. They are widely used as reagents in organic chemistry. For example, benzyl chloroformate is used to introduce the CBZ protecting group and fluorenylmethyloxycarbonylchloride is used to introduce the FMOC...
quench to triene 3 which was then reacted in a Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
(benzene 185°C) to hexene 4. The three ester groups were hydrolyzed using iodotrimethylsilane forming pentacyclic lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
5 after a methanol quench in a combination of 7 reaction steps (one of them a Dieckmann condensation
Dieckmann condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-ketoesters. It is named after the German chemist Walter Dieckmann . The equivalent intermolecular reaction is the Claisen condensation....
). The C4 segment 6 was added in an amine alkylation
Amine alkylation
Amine alkylation is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution , and the reaction product is a higher substituted amine...
and Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
of 7 formed isostrychnine 8 after TBS deprotection.
Bosch synthesis
In the Bosch synthesis of (1999, chiral) the olefin group in dione 1 was converted to an aldehydeAldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
by ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
and chiral amine 2 was formed in a double reductive amination
Reductive amination
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine...
with (S)-1-phenylethylamine. The phenylethyl substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
was removed using ClCO2CHClCH3 and the enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
group was introduced in a Grieco elimination
Grieco elimination
The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene ....
using TMSI, HMDS
Bis(trimethylsilyl)amine
Bisamine is an organosilicon compound with the molecular formula [3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms...
then PhSeCl then ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
and then diisopropylamine
Diisopropylamine
Diisopropylamine is a secondary amine with the chemical formula 2HC-NH-CH2. It is best known as its lithium salt, lithium diisopropylamide, known as "LDA"...
forming carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
3. The amino group was deprotected by refluxing in methanol and then alkylated
Amine alkylation
Amine alkylation is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution , and the reaction product is a higher substituted amine...
using (Z)-BrCH2CICH=CH2OTBDMS, to tertiary amine 4. A reductive Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
took place next followed by methoxycarbonylation (LiHMDS, NCCO2Me) to tricycle 5. Reaction with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
dust in 10% sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
removed the TBDMS protective group, reduced the nitro group and brought about a reductive amino-carbonyl cyclization in a single step to tetracyclic 6 (epimeric mixture). In the final step to the Wieland-Gumlich aldehyde
Wieland-Gumlich aldehyde
The Wieland-Gumlich aldehyde is an indoline derived from strychnine. This compound is of some commercial interest as a chemical intermediate. It was first synthesized in 1932 by Wieland and Gumlich in 4 steps from strychnine...
7 reaction with NaH
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
in MeOH afforded the correct epimer was followed by DIBAH reduction of the methyl ester.
Vollhardt synthesis
The key reaction in the Vollhardt synthesis (2000, racemic) was an alkyne trimerisationAlkyne trimerisation
An alkyne trimerisation reaction is a 2+2+2 cyclization reaction in which three alkyne molecules react to form an aromatic compound. The reaction is 'pseudo' pericyclic since it has not been observed to occur without the assistance of metal catalysis; and the metal catalyst assembles the ring...
of tryptamine
Tryptamine
Tryptamine is a monoamine alkaloid found in plants, fungi, and animals. It is based around the indole ring structure, and is chemically related to the amino acid tryptophan, from which its name is derived...
derivative 1 with acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
and organocobalt compound CpCo(C2H4)2 (THF, 0 °C) to tricycle 2 after deprotection of the amine group (KOH, MeOH/H2O reflux). Subsequent reaction with iron nitrate brought about a [1,8]-conjugate addition to tetracycle 3, amine alkylation
Amine alkylation
Amine alkylation is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution , and the reaction product is a higher substituted amine...
with (Z)-1-bromo-4-[(tert-butyldimethylsilyl)oxy]-2-iodobut-2-ene (see Rawal synthesis) and lithium carbonate
Lithium carbonate
Lithium carbonate is a chemical compound of lithium, carbon, and oxygen with the formula Li2CO3. This colorless salt is widely used in the processing of metal oxides and has received attention for its use in psychiatry. It is found in nature as the rare mineral zabuyelite.-Properties:Like almost...
, and isomerization of the diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
system (NaOiPr, iPrOH) formed enone 4. A Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
as in the Rawal synthesis (palladium acetate / triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
), accompanied by aromatization formed pyridone 5 and lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
reduction and TBS group deprotection formed isostrychnine 6.
Mori synthesis
The Mori synthesis ((-) chiral, 2003) was the first one containing an asymmetric reaction step. It also features a large number of Pd catalyzed reactions. In it N-tosyl amine 1 reacted with allyl carbonate 2 in an allylic asymmetric substitution using Pd2(dba)3Tris(dibenzylideneacetone)dipalladium(0)
Trisdipalladium or Pd23 is an organometallic complex based on palladium and dibenzylideneacetone used in organic chemistry. It was discovered in 1970.-Preparation and structure:...
and asymmetric ligand (S-BINAPO) to chiral secondary amine 3. Desilylation of the TBDMS group next took place by HCl
HCL
HCL or HCl can stand for:* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia* Hardware compatibility list...
to the hydroxide
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
and then to the nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
4 (NaCN) through the bromide (PBr3
Phosphorus tribromide
Phosphorus tribromide is a colourless liquid with the formula PBr3. It fumes in air due to hydrolysis and has a penetrating odour. It is widely used in the laboratory for the conversion of alcohols to alkyl bromides.-Preparation:...
). Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
(Pd(OAc)2 / Me2PPh
Dimethylphenylphosphine
Dimethylphenylphosphine is a organophosphorus compound with a formula P2. The phosphorus is connected to a phenyl group and two methyl groups. This colorless air sensitive liquid is commonly used as a ligand in transition metal complexes...
) and debromination (Ag2CO3
Silver carbonate
Silver carbonate is the chemical compound with the formula Ag2CO3. Silver carbonate is yellow but typical samples are grayish due to the presence of elemental silver. It is poorly soluble in water, like most transition metal carbonates. Silver carbonate is used as a reagent in organic synthesis...
) afforded tricycle 5. LiALH4
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
Nitrile reduction
Nitrile reduction
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent .Reagents for the conversion to amines are lithium aluminium hydride, Raney nickel / hydrogen / or diborane This organic reaction is one of several nitrogen-hydrogen bond forming...
to the amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
and its Boc2O
Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. This carbonate ester reacts with amines to give N-tert-butoxycarbonyl or so-called t-BOC derivatives. These derivatives do not behave as amines, which allows certain subsequent transformations to occur that would have...
protection to boc amine 6 was then followed by a second allylic oxidation (Pd(OAc)2 / AcOH
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
/ benzoquinone / MnO2
Manganese oxide
Manganese oxide is a generic term used to describe a variety of manganese oxides and hydroxides. It may refer to:* Manganese oxide, MnO* Manganese oxide, Mn3O4* Manganese oxide, Mn2O3* Manganese dioxide, , MnO2...
) to tetracycle 7. Hydroboration-oxidation (9-BBN
9-BBN
9-Borabicyclo[3.3.1]nonane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates...
/ H2O2
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
) gave alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
8 and subsequent Swern oxidation
Swern oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide and an organic base, such as triethylamine...
ketone 9. Reaction with LDA
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
/ PhNTf2 gave enol triflate 10 and the triflate group was removed in alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
11 by reaction with Pd(OAc)2 and PPh3
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
.
Detosylation of 11 (sodium naphthalenide
Sodium naphthalenide
Sodium naphthalenide, also known as sodium naphthalide, is a soluble one-electron reductant employed in organic, organometallic, and inorganic chemistry. It is prepared by stirring sodium metal with naphthalene in a polar solvent such as tetrahydrofuran or dimethoxyethane, resulting in the...
) and amidation with acid chloride 3-bromoacryloyl chloride gave amide 12 and another Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
gave pentacycle 13. double bond isomerization (sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
/ iPrOH
Isopropyl alcohol
Isopropyl alcohol is a common name for a chemical compound with the molecular formula C3H8O. It is a colorless, flammable chemical compound with a strong odor...
), Boc group deprotection (triflic acid) and amine alkylation
Amine alkylation
Amine alkylation is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution , and the reaction product is a higher substituted amine...
with (Z)-BrCH2CICH=CH2OTBDMS (see Rawal) gave compound 14 (identical to one of the Vollhardt intermediates). A final heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
(15) and TBDMS deprotection formed (-)-isostrychnine 16.
Shibasaki synthesis
The Shibasaki synthesis ((-) chiral, 2002) was a second published method in strychnine total synthesis using an asymmetric reaction step. CyclohexenoneCyclohexenone
Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances...
1 was reacted with dimethyl malonate
Dimethyl malonate
Dimethyl malonate is a diester derivative of malonic acid. It is a common reagent for organic synthesis. It is used in the malonic ester synthesis....
2 in an asymmetric Michael reaction using AlLibis(binaphthoxide) to form chiral diester 3. It's ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
group was protected as an acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
(2-ethyl-2-methyl-1,3-dioxolane, TsOH) and a carboxyl group was removed (LiCl, DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
140 °C) in monoester 4. A C2 fragment was added as Weinreb amide 5 to form PMB ether 6 using LDA
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
. The ketone was then reduced to the alcohol (NaBH3CN
Sodium cyanoborohydride
Sodium cyanoborohydride is the inorganic compound with the formula NaBH3. This colourless salt is widely used in organic synthesis for the reduction of imines.-Preparation and use:...
, TiCl4
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
) and then water was eliminated
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
(DCC, CuCl
Copper(I) chloride
Copper chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid...
) to form alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
7. After ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
reduction (DIBAL) to the alcohol and its TIPS protection (TIPSOTf, triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
), the acetal group was removed (catalytic CSA
Camphorsulfonic acid
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is a organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that exists as a colourless solid that is soluble in organic solvents....
) in ketone 8. Enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
9 was then formed by Saegusa oxidation. The conversion to alcohol 10 was accomplished via a Mukaiyama aldol addition
Mukaiyama aldol addition
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde catalyzed by a Lewis acid. This choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone or a different aldehyde without self-condensation of...
using formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
, iodonation to 11 (iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, DMAP) was followed by a Stille coupling (Pd2dba3
Tris(dibenzylideneacetone)dipalladium(0)
Trisdipalladium or Pd23 is an organometallic complex based on palladium and dibenzylideneacetone used in organic chemistry. It was discovered in 1970.-Preparation and structure:...
, Ph3As
Triphenylarsine
Triphenylarsine is the chemical compound with the formula As3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis...
, CuI
Cui
-People:* Cui, Chinese surname.* César Cui , a Russian composer* Jorge "Cui" Cuello, British historian Oxford University, 1995 Pulitzer Prize winner-Other uses:* Cui , a Peruvian term for the guinea pig, when used as food...
) incorporating nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
unit 12. Alcohol 13 was formed after SEM
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
protection (SEMCl,i-Pr2NEt) and TIPS removal (HF
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
).
In the second part of the sequence alcohol 13 was converted to a triflate (Triflic anhydride
Triflic anhydride
Triflic anhydride is the chemical compound with the formula 2O. This compound is a particularly strong electrophile, useful for introducing the triflyl group, CF3SO2. Triflic anhydride is the acid anhydride of the strong acid triflic acid, CF3SO2OH....
, N,N-Diisopropylethylamine), then 2,2-bis(ethylthio)ethylamine 14 was added immediately followed by zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
powder, setting of a tandem reaction with nitro group reduction to the amine, 1,4-addition of the thio-amine group and amine-keto condensation to indole 16. Reaction with DMTSF gave thionium attack at C7 forming 17, the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
group was then reduced (NaBH3CN
Sodium cyanoborohydride
Sodium cyanoborohydride is the inorganic compound with the formula NaBH3. This colourless salt is widely used in organic synthesis for the reduction of imines.-Preparation and use:...
, TiCl4
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
), the new amino group acylated
Acylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
(acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
), both alcohol protecting groups removed (NaOMe / meOH) and the allyl alcohol group protected again (TIPS). This allowed removal of the ethylthio group (NiCl2
Nickel(II) chloride
Nickel chloride , is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. It is very rarely found in nature as mineral nickelbischofite. A dihydrate is also known. In general nickel chloride, in various forms, is the most important source of...
, NaBH4
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
, EtOH/MeOH) to 18. The alcohol was oxidized to the aldehyde using a Parikh-Doering oxidation
Parikh-Doering oxidation
The Parikh-Doering oxidation is an oxidation reaction that transforms primary and secondary alcohols into aldehydes and ketones, respectively. The procedure uses DMSO as the oxidant, activated by sulfur trioxide-pyridine complex in the presence of triethylamine base:The procedure can be run at...
and TIPS group removal gave hemiacetal 19 called (+)-diaboline which is acylated Wieland-Gumlich aldehyde
Wieland-Gumlich aldehyde
The Wieland-Gumlich aldehyde is an indoline derived from strychnine. This compound is of some commercial interest as a chemical intermediate. It was first synthesized in 1932 by Wieland and Gumlich in 4 steps from strychnine...
.
Li synthesis
The synthesis reported by Bodwell/Li(racemic, 2002) was a formal synthesis as it produced a compound already prepared by Rawal (no. 5 in the Rawal synthesis). The key step was an inverse-electron-demand Diels-Alder reaction of cyclophaneCyclophane
A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known...
1 by heating in N,N-diethylaniline (dinitrogen is expulsed) followed by reduction of double bond in 2 to 3 by sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
/ triflic acid and removal of the carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
(PDC / celite) to 4.
The method is disputed by Reissig (see Reissig synthesis).
Fukuyama synthesis
The Fukuyama synthesis (chiral (-), 2004) started from cyclic amine 1. Chirality was at some point introduced into this starting material by enzymatic resolution of one of the precursors. AcyloinAcyloin
Acyloins are a class of organic compounds in organic chemistry sharing a common functional group consisting of a hydroxyl group placed on the α-position of a carbonyl group.- Nomenclature :Common types of ketols include:...
2 was formed by Rubottom oxidation
Rubottom oxidation
The Rubottom oxidation is the chemical reaction of enolsilanes with m-chloroperoxybenzoic acid to give silyl-protected α-hydroxy ketones.-Reaction mechanism:...
and hydrolysis. Oxidative cleavage by lead acetate
Lead acetate
Lead acetate can refer to:* Lead acetate , Pb4* Lead acetate , Pb2...
formed aldehyde 3, removal of the nosyl group (thiophenol
Thiophenol
Thiophenol is an organosulfur compound with the formula C6H6S, and sometimes abbreviated as PhSH. This foul-smelling colourless liquid is the simplest aromatic thiol. The chemical structures of thiophenols are analogous to phenols except the oxygen atom in the hydroxyl group bonded to the...
/ cesium carbonate) triggered an amine-carbonyl condensation with iminium ion 4 continuing to react in a transannular cyclization to diester 5 which could be converted to the Wieland-Gumlich aldehyde
Wieland-Gumlich aldehyde
The Wieland-Gumlich aldehyde is an indoline derived from strychnine. This compound is of some commercial interest as a chemical intermediate. It was first synthesized in 1932 by Wieland and Gumlich in 4 steps from strychnine...
by known chemistry.
Reissig synthesis
The method reported by Beemelmanns & Reissig (racemic, 2010) is another formal synthesis leading to the Rawal pentacycle (see amine 5 in the Rawal method). In this method indole 1 was converted to tetracycle 2 (together with by-product) in a single cascade reactionCascade reaction
A cascade reaction or tandem reaction or domino reaction is a consecutive series of intramolecular organic reactions which often proceed via highly reactive intermediates. It allows the organic synthesis of complex multinuclear molecules from a single acyclic precursor. The substrate contains many...
using samarium diiodide and HMPA. Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
/ H2 reduction gave amine 3 and a one-pot reaction using methyl chloroformate
Chloroformate
Chloroformates are a class of chemical compounds which are esters of chloroformic acid. They are widely used as reagents in organic chemistry. For example, benzyl chloroformate is used to introduce the CBZ protecting group and fluorenylmethyloxycarbonylchloride is used to introduce the FMOC...
, DMAP and TEA
Tea
Tea is an aromatic beverage prepared by adding cured leaves of the Camellia sinensis plant to hot water. The term also refers to the plant itself. After water, tea is the most widely consumed beverage in the world...
then MsCl, DMAP and TEA
Triethanolamine
Triethanolamine, often abbreviated as TEA, is an organic chemical compound which is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Like other amines, triethanolamine is a strong base due to the lone pair of electrons on the nitrogen atom. Triethanolamine can...
and then DBU gave Rawal precursor 4 with key hydrogen atoms in the desired anti configuration.
In an aborted route intermediate 2 was first reduced to imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
5 then converted to carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
6, then dehydrated to diene 7 (Burgess reagent
Burgess reagent
The Burgess reagent or methyl N-carbamate was developed in the laboratory of Edward M. Burgess at Georgia Tech. It is a mild and selective dehydrating reagent often used in organic chemistry. It is used to convert secondary and tertiary alcohol with an adjacent proton into alkenes. Primary alcohols...
) and finally reduced to 8 (sodium cyanoborohydride
Sodium cyanoborohydride
Sodium cyanoborohydride is the inorganic compound with the formula NaBH3. This colourless salt is widely used in organic synthesis for the reduction of imines.-Preparation and use:...
). The hydrogen atoms in 8 are in an undesired cis-relationship which contradicts the results obtained in 2002 by Bodwell/Li for the same reaction.
Vanderwal synthesis
In 2011, Vanderwal group reported a concise, longest linear sequence of 6 steps, total synthesis of strychnine. It featured a Zincke reactionZincke reaction
The Zincke reaction is an organic reaction in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine, named after Theodor Zincke....
followed by an anionic bicyclization reaction and a tandem Brook rearrangement
Brook rearrangement
The Brook rearrangement in organic chemistry is a rearrangement reaction in which a organosilyl group switches position with a hydroxyl proton over a carbon to oxygen covalent bond under the influence of a base . It is named for the Canadian chemist Adrian Gibbs Brook...
/ conjugate addition.

