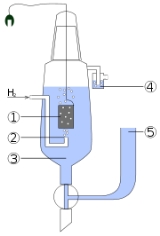
Standard hydrogen electrode
Overview
Absolute electrode potential
Absolute electrode potential, in electrochemistry, according to an IUPAC definition, is the electrode potential of a metal measured with respect to a universal reference system .-Definition:...
is estimated to be 4.44 ± 0.02 V at 25 °C, but to form a basis for comparison with all other electrode reactions, hydrogen's standard electrode potential
Standard electrode potential
In electrochemistry, the standard electrode potential, abbreviated E° or E , is the measure of individual potential of a reversible electrode at standard state, which is with solutes at an effective concentration of 1 mol dm−3, and gases at a pressure of 1 atm...
(E0) is declared to be zero at all temperatures. Potentials of any other electrodes are compared with that of the standard hydrogen electrode at the same temperature.
Hydrogen electrode is based on the redox half cell
Half cell
A half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electric charges between the electrode and the electrolyte, resulting in a...
:
- 2H+(aq) + 2e- → H2(g)
This redox reaction occurs at platinized platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
electrode.
The electrode is dipped in an acidic solution and pure hydrogen gas is bubbled through it.
Unanswered Questions

