
Standard enthalpy change of combustion
Encyclopedia
The standard enthalpy of combustion is the enthalpy
change when one mole
of a substance completely reacts with oxygen under standard thermodynamic conditions
(although experimental values are usually obtained under different conditions and subsequently adjusted). By definition, combustion reactions are always exothermic
and so enthalpies of combustion are always negative, although the values for individual combustions may vary.
It is commonly denoted as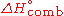 or
or 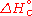 .
.
When the enthalpy required is not a combustion, it can be denoted as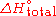 .
.
Enthalpies of combustion are typically measured using bomb calorimetry
, and have units of energy (typically kJ); strictly speaking, the enthalpy change per mole of substance combusted is the standard molar enthalpy of combustion (which typically would have units of kJ mol−1).
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change when one mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of a substance completely reacts with oxygen under standard thermodynamic conditions
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
(although experimental values are usually obtained under different conditions and subsequently adjusted). By definition, combustion reactions are always exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
and so enthalpies of combustion are always negative, although the values for individual combustions may vary.
It is commonly denoted as
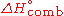 or
or 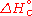 .
. When the enthalpy required is not a combustion, it can be denoted as
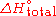 .
.Enthalpies of combustion are typically measured using bomb calorimetry
Calorimeter
A calorimeter is a device used for calorimetry, the science of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal microcalorimeters, titration calorimeters and accelerated rate calorimeters are among the most common...
, and have units of energy (typically kJ); strictly speaking, the enthalpy change per mole of substance combusted is the standard molar enthalpy of combustion (which typically would have units of kJ mol−1).

