
Solid solution strengthening
Encyclopedia
Solid solution strengthening is a type of alloy
ing that can be used to improve the strength of a pure metal. The technique works by adding atoms of one element (the alloying element) to the crystalline lattice of another element (the base metal). The alloying element diffuses into the matrix, forming a solid solution
. In most binary systems, when alloyed above a certain concentration, a second phase will form. When this increases the strength of the material, the process is known as precipitation strengthening
, but this is not always the case.
Substitutional solid solution strengthening occurs when the solute atom is large enough that it can replace solvent atoms in their lattice positions. According to the Hume-Rothery rules
, solvent and solute atoms must differ in atomic size by less than 15% in order to form this type of solution. Because both elements exist in the same crystalline lattice, both elements in their pure form must be of the same crystal structure
. Examples of substitutional solid solutions include the Cu-Ni and the Ag-Au FCC binary systems, and the Mo-W BCC binary system.
When the solute atom is much smaller than the solvent atoms, an interstitial solid solution forms. This typically occurs when the solute atoms are less than half as small as the solvent atoms. The smaller solute atom essentially "crowds" into the spacings within the lattice structure, causing defects in the material. Elements commonly used to form interstitial solid solutions include H, N, C, and O. Carbon in iron (steel) is one example of interstitial solid solution.
When solute and solvent atoms differ in size, local stress fields are created (if solute atom size is larger than solvent atom size, this field is compressive, and similarly, when solute atoms are smaller than solvent atoms, this field is tensile). Depending on their relative locations, solute atoms will either attract or repel dislocations in their vicinity. This is known as the size effect. This allows the solute atoms to relieve either tensile or compressive strain in the lattice, which in turn puts the dislocation in a lower energy state. In substitutional solid solutions, these stress fields are spherically symmetric, meaning they have no shear stress component. As such, substitutional solute atoms do not interact with the shear stress fields characteristic of screw dislocations. Conversely, in interstitial solid solutions, solute atoms cause a tetragonal distortion, generating a shear field that can interact with both edge, screw, and mixed dislocations. The attraction or repulsion of the dislocation centers to the solute particles increase the stress it takes to propagate the dislocation in any other direction. Increasing the applied stress to move the dislocation increases the yield strength of the material.
The energy density of a dislocation
is dependent on its Burgers vector
as well as the modulus of the local atoms. When the modulus of solute atoms differs from that of the host element, the local energy around the dislocation is changed, increasing the amount of force necessary to move past this energy well. This is known as the modulus effect. Meanwhile, in the specific case of a lattice distortion, the difference in lattice parameter leads to a high stress field around that solute atom that impedes dislocation movement.
Surface carburizing, or case hardening
, is one example of solid solution strengthening in which the density of solute carbon atoms is increased close to the surface of the steel, resulting in a gradient of carbon atoms throughout the material. This provides superior mechanical properties to the surface of the steel.
 to move dislocations:
to move dislocations:
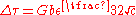
where c is the concentration of the solute atoms, G is the shear modulus, b is the magnitude of the Burger's vector, and is the lattice strain due to the solute. This is composed of two terms, one describing lattice distortion and the other local modulus change.
is the lattice strain due to the solute. This is composed of two terms, one describing lattice distortion and the other local modulus change.
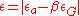
Here,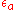 is the lattice distortion term,
is the lattice distortion term, 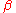 a constant dependent on the solute atoms and
a constant dependent on the solute atoms and  the term that captures the local modulus change.
the term that captures the local modulus change.
The lattice distortion term can be described as:
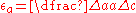 , where a is the lattice parameter of the material.
, where a is the lattice parameter of the material.
Meanwhile, the local modulus change is captured in the following expression:
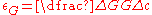 , where G is shear modulus of the solute material,
, where G is shear modulus of the solute material,
Alloying with elements of higher shear modulus or of very different lattice parameters will increase the stiffness and introduce local stress fields respectively. In either case, the dislocation propagation will be hindered at these sites, impeding plasticity and increasing yield strength proportionally with solute concentration.
Solid solution strengthening depends on:
- Concentration of solute atoms
- Shear modulus of solute atoms
- Size of solute atoms
- Valency of solute atoms (for ionic materials)
Nevertheless, one should not add so much solute as to precipitate a new phase. This occurs if the concentration of the solute reaches a high critical point given by the binary system phase diagram. This critical concentration therefore puts a limit to the amount of solid solution strengthening a material can have, as the material cannot be infinitely strengthened.
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
ing that can be used to improve the strength of a pure metal. The technique works by adding atoms of one element (the alloying element) to the crystalline lattice of another element (the base metal). The alloying element diffuses into the matrix, forming a solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
. In most binary systems, when alloyed above a certain concentration, a second phase will form. When this increases the strength of the material, the process is known as precipitation strengthening
Precipitation strengthening
Precipitation hardening, also called age hardening, is a heat treatment technique used to increase the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel and titanium, and some stainless steels...
, but this is not always the case.
Types
Depending on the size of the alloying element, a substitutional solid solution or an interstitial solid solution can form. In both cases, the overall crystal structure is essentially unchanged.Substitutional solid solution strengthening occurs when the solute atom is large enough that it can replace solvent atoms in their lattice positions. According to the Hume-Rothery rules
Hume-Rothery rules
The Hume-Rothery rules, named after William Hume-Rothery, are a set of basic rules describing the conditions under which an element could dissolve in a metal, forming a solid solution...
, solvent and solute atoms must differ in atomic size by less than 15% in order to form this type of solution. Because both elements exist in the same crystalline lattice, both elements in their pure form must be of the same crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
. Examples of substitutional solid solutions include the Cu-Ni and the Ag-Au FCC binary systems, and the Mo-W BCC binary system.
When the solute atom is much smaller than the solvent atoms, an interstitial solid solution forms. This typically occurs when the solute atoms are less than half as small as the solvent atoms. The smaller solute atom essentially "crowds" into the spacings within the lattice structure, causing defects in the material. Elements commonly used to form interstitial solid solutions include H, N, C, and O. Carbon in iron (steel) is one example of interstitial solid solution.
Mechanism
The strength of a material is dependent on how easily dislocations in its crystal lattice can be propagated. These dislocations create stress fields within the material depending on their character. When solute atoms are introduced, local stress fields are formed that interact with those of the dislocations, impeding their motion and causing an increase in the yield stress of the material, which means an increase in strength of the material. This gain is a result of both lattice distortion and the modulus effect.When solute and solvent atoms differ in size, local stress fields are created (if solute atom size is larger than solvent atom size, this field is compressive, and similarly, when solute atoms are smaller than solvent atoms, this field is tensile). Depending on their relative locations, solute atoms will either attract or repel dislocations in their vicinity. This is known as the size effect. This allows the solute atoms to relieve either tensile or compressive strain in the lattice, which in turn puts the dislocation in a lower energy state. In substitutional solid solutions, these stress fields are spherically symmetric, meaning they have no shear stress component. As such, substitutional solute atoms do not interact with the shear stress fields characteristic of screw dislocations. Conversely, in interstitial solid solutions, solute atoms cause a tetragonal distortion, generating a shear field that can interact with both edge, screw, and mixed dislocations. The attraction or repulsion of the dislocation centers to the solute particles increase the stress it takes to propagate the dislocation in any other direction. Increasing the applied stress to move the dislocation increases the yield strength of the material.
The energy density of a dislocation
Dislocation
In materials science, a dislocation is a crystallographic defect, or irregularity, within a crystal structure. The presence of dislocations strongly influences many of the properties of materials...
is dependent on its Burgers vector
Burgers vector
The Burgers vector, named after Dutch physicist Jan Burgers, is a vector, often denoted b, that represents the magnitude and direction of the lattice distortion of dislocation in a crystal lattice....
as well as the modulus of the local atoms. When the modulus of solute atoms differs from that of the host element, the local energy around the dislocation is changed, increasing the amount of force necessary to move past this energy well. This is known as the modulus effect. Meanwhile, in the specific case of a lattice distortion, the difference in lattice parameter leads to a high stress field around that solute atom that impedes dislocation movement.
Surface carburizing, or case hardening
Case hardening
Case hardening or surface hardening is the process of hardening the surface of a metal, often a low carbon steel, by infusing elements into the material's surface, forming a thin layer of a harder alloy...
, is one example of solid solution strengthening in which the density of solute carbon atoms is increased close to the surface of the steel, resulting in a gradient of carbon atoms throughout the material. This provides superior mechanical properties to the surface of the steel.
Governing equations
Solid solution strengthening increases yield strength of the material by increasing the stress to move dislocations:
to move dislocations: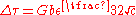
where c is the concentration of the solute atoms, G is the shear modulus, b is the magnitude of the Burger's vector, and
 is the lattice strain due to the solute. This is composed of two terms, one describing lattice distortion and the other local modulus change.
is the lattice strain due to the solute. This is composed of two terms, one describing lattice distortion and the other local modulus change.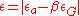
Here,
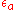 is the lattice distortion term,
is the lattice distortion term, 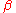 a constant dependent on the solute atoms and
a constant dependent on the solute atoms and  the term that captures the local modulus change.
the term that captures the local modulus change.The lattice distortion term can be described as:
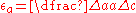 , where a is the lattice parameter of the material.
, where a is the lattice parameter of the material.Meanwhile, the local modulus change is captured in the following expression:
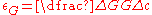 , where G is shear modulus of the solute material,
, where G is shear modulus of the solute material,Implications
In order to achieve noticeable material strengthening via solute solution strengthening one should alloy with solutes of higher shear modulus, hence increasing the local shear modulus in the material. In addition, one should alloy with elements of different equilibrium lattice constants. The greater the different in lattice parameter, the higher the local stress fields introduced by alloying.Alloying with elements of higher shear modulus or of very different lattice parameters will increase the stiffness and introduce local stress fields respectively. In either case, the dislocation propagation will be hindered at these sites, impeding plasticity and increasing yield strength proportionally with solute concentration.
Solid solution strengthening depends on:
- Concentration of solute atoms
- Shear modulus of solute atoms
- Size of solute atoms
- Valency of solute atoms (for ionic materials)
Nevertheless, one should not add so much solute as to precipitate a new phase. This occurs if the concentration of the solute reaches a high critical point given by the binary system phase diagram. This critical concentration therefore puts a limit to the amount of solid solution strengthening a material can have, as the material cannot be infinitely strengthened.

