Silicon burning process
Encyclopedia
In astrophysics
, silicon
burning is a very brief sequence of nuclear fusion
reactions that occur in massive star
s with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main sequence
on the Hertzsprung-Russell diagram. It follows the previous stages of hydrogen
, helium
(the triple-alpha process
), carbon
, neon
and oxygen
burning processes.
Silicon burning begins when gravitational contraction raises the star’s core temperature to 2.7–3.5 billion kelvins (GK). The exact temperature depends on mass. When a star has completed the silicon-burning phase, no further fusion is possible. The star catastrophically collapses and may explode in what is known as a Type II
supernova
.
in their cores has been consumed and fused into helium
. Stars with an intermediate mass (greater than 3 but less than about 8-11 solar masses) can go on to "burn" (fuse) helium into carbon by means of the triple-alpha process
. These stars end their lives when the helium in their cores has been exhausted; thus they end up with carbon cores. High mass stars (more than about 8–11 solar masses) are able to burn carbon because of the extraordinarily high gravitational potential energy
bound in their mass. As massive stars contract, their cores heat up to 600 MK and carbon burning
begins which creates new elements
as follows:
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|}
The chemical elements are defined by the number of proton
s in their nucleus. In the elements listed above, the superscript denotes a particular isotope
(form of a chemical element having a different number of neutron
s) in terms of its molar mass
.
After a high-mass star has burned all the carbon in its core, it contracts, gets hotter, and begins burning the oxygen, neon, and magnesium as follows:
|- style="height:2em;"
| ||+ || ||→ ||
|}
After high-mass stars have nothing but sulfur and silicon in their cores, they further contract until their cores reach temperatures in the range of 2.7–3.5 GK (230–300 keV
); silicon burning starts at this point. Silicon burning entails the alpha process which creates new elements by adding the equivalent of one helium nucleus (two protons plus two neutrons) per step in the following sequence:
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ |||| || (energy is consumed and the star's core collapses)
|}
The entire silicon-burning sequence lasts about one day and stops when nickel–56 has been produced. Nickel–56 (which has 28 protons) has a half-life
of 6.02 days and decays via beta radiation
(in this case, "beta-plus" decay, which is the emission of a positron) to cobalt
–56 (27 protons), which in turn has a half-life of 77.3 days as it decays to iron-56
(26 protons). However, only minutes are available for the nickel–56 to decay within the core of a massive star. At the end of the day-long silicon-burning sequence, the star can no longer release energy via nuclear fusion because a nucleus with 56 nucleons has the lowest mass per nucleon
(proton and neutron) of all the elements in the alpha process sequence. Although iron–58 and nickel–62 have slightly higher binding energies per nucleon than iron–56, the next step up in the alpha process would be zinc
–60, which has slightly more mass per nucleon and thus, would actually consume energy in its production rather than release any. The star has run out of nuclear fuel and within minutes begins to contract. The potential energy of gravitational contraction heats the interior to 5 GK/430 keV and this opposes and delays the contraction. However, since no additional heat energy can be generated via new fusion reactions, the contraction rapidly accelerates into a collapse lasting only a few seconds. The central portion of the star gets crushed into either a neutron star
or, if the star is massive enough, a black hole
. The outer layers of the star are blown off in an explosion known as a Type II
supernova
that lasts days to months. The supernova explosion releases a large burst of neutrons, which synthesizes in about one second roughly half the elements heavier than iron, via a neutron-capture mechanism known as the r-process
(where the “r” stands for rapid neutron capture).
. In stars, rapid nucleosynthesis proceeds by adding helium nuclei (alpha particles) to heavier nuclei. Although nuclei with 58 and 62 nucleons have the very lowest binding energy, fusing a helium nucleus into nickel–56 (14 alphas) to produce the next element—zinc–60 (15 alphas)—actually requires energy rather than releases any. Accordingly, nickel–56 is the last fusion product produced in the core of a high-mass star. Decay of nickel-56 explains the large amount of iron-56 seen in metallic meteorites and the cores of rocky planets.
Astrophysics
Astrophysics is the branch of astronomy that deals with the physics of the universe, including the physical properties of celestial objects, as well as their interactions and behavior...
, silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
burning is a very brief sequence of nuclear fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
reactions that occur in massive star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main sequence
Main sequence
The main sequence is a continuous and distinctive band of stars that appears on plots of stellar color versus brightness. These color-magnitude plots are known as Hertzsprung–Russell diagrams after their co-developers, Ejnar Hertzsprung and Henry Norris Russell...
on the Hertzsprung-Russell diagram. It follows the previous stages of hydrogen
Hydrogen burning process
In the context of stellar nucleosynthesis, a hydrogen-burning process can refer to either the proton-proton chain reactions dominant in main sequence stars lighter than at most 5 solar masses, or to the CNO cycle dominant in heavier stars. Both processes produces stellar energy by burning hydrogen...
, helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
(the triple-alpha process
Triple-alpha process
The triple alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei are transformed into carbon.Older stars start to accumulate helium produced by the proton–proton chain reaction and the carbon–nitrogen–oxygen cycle in their cores...
), carbon
Carbon burning process
The carbon-burning process or carbon fusion is a set of nuclear fusion reactions that take place in massive stars that have used up the lighter elements in their cores...
, neon
Neon burning process
The neon-burning process is a set of nuclear fusion reactions that take place in massive stars . Neon burning requires high temperatures and densities ....
and oxygen
Oxygen burning process
The oxygen-burning process is a set of nuclear fusion reactions that take place in massive stars that have used up the lighter elements in their cores. It occurs at temperatures around 1.5×109 K / 130 keV and densities of 1010 kg/m3....
burning processes.
Silicon burning begins when gravitational contraction raises the star’s core temperature to 2.7–3.5 billion kelvins (GK). The exact temperature depends on mass. When a star has completed the silicon-burning phase, no further fusion is possible. The star catastrophically collapses and may explode in what is known as a Type II
Type II supernova
A Type II supernova results from the rapid collapse and violent explosion of a massive star. A star must have at least 9 times, and no more than 40–50 times the mass of the Sun for this type of explosion. It is distinguished from other types of supernova by the presence of hydrogen in its spectrum...
supernova
Supernova
A supernova is a stellar explosion that is more energetic than a nova. It is pronounced with the plural supernovae or supernovas. Supernovae are extremely luminous and cause a burst of radiation that often briefly outshines an entire galaxy, before fading from view over several weeks or months...
.
Nuclear fusion sequence and the alpha process
Stars with normal mass (no greater than about 3 solar masses) run out of fuel after the hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
in their cores has been consumed and fused into helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
. Stars with an intermediate mass (greater than 3 but less than about 8-11 solar masses) can go on to "burn" (fuse) helium into carbon by means of the triple-alpha process
Triple-alpha process
The triple alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei are transformed into carbon.Older stars start to accumulate helium produced by the proton–proton chain reaction and the carbon–nitrogen–oxygen cycle in their cores...
. These stars end their lives when the helium in their cores has been exhausted; thus they end up with carbon cores. High mass stars (more than about 8–11 solar masses) are able to burn carbon because of the extraordinarily high gravitational potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
bound in their mass. As massive stars contract, their cores heat up to 600 MK and carbon burning
Carbon burning process
The carbon-burning process or carbon fusion is a set of nuclear fusion reactions that take place in massive stars that have used up the lighter elements in their cores...
begins which creates new elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
as follows:
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|}
The chemical elements are defined by the number of proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s in their nucleus. In the elements listed above, the superscript denotes a particular isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
(form of a chemical element having a different number of neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s) in terms of its molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
.
After a high-mass star has burned all the carbon in its core, it contracts, gets hotter, and begins burning the oxygen, neon, and magnesium as follows:
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ ||
|}
After high-mass stars have nothing but sulfur and silicon in their cores, they further contract until their cores reach temperatures in the range of 2.7–3.5 GK (230–300 keV
Kev
Kev can refer to:*Kev Hawkins, a fictional character.*Kevin, a given name occasionally shortened to "Kev".*Kiloelectronvolt, a unit of energy who symbol is "KeV".* Krefelder Eislauf-VereinKEV can refer to:...
); silicon burning starts at this point. Silicon burning entails the alpha process which creates new elements by adding the equivalent of one helium nucleus (two protons plus two neutrons) per step in the following sequence:
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ |||| || (energy is consumed and the star's core collapses)
|}
The entire silicon-burning sequence lasts about one day and stops when nickel–56 has been produced. Nickel–56 (which has 28 protons) has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 6.02 days and decays via beta radiation
Beta particle
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay...
(in this case, "beta-plus" decay, which is the emission of a positron) to cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
–56 (27 protons), which in turn has a half-life of 77.3 days as it decays to iron-56
Iron-56
Iron-56 is the most common isotope of iron. About 91.754% of all iron is iron-56.Of all isotopes, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei....
(26 protons). However, only minutes are available for the nickel–56 to decay within the core of a massive star. At the end of the day-long silicon-burning sequence, the star can no longer release energy via nuclear fusion because a nucleus with 56 nucleons has the lowest mass per nucleon
Nucleon
In physics, a nucleon is a collective name for two particles: the neutron and the proton. These are the two constituents of the atomic nucleus. Until the 1960s, the nucleons were thought to be elementary particles...
(proton and neutron) of all the elements in the alpha process sequence. Although iron–58 and nickel–62 have slightly higher binding energies per nucleon than iron–56, the next step up in the alpha process would be zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
–60, which has slightly more mass per nucleon and thus, would actually consume energy in its production rather than release any. The star has run out of nuclear fuel and within minutes begins to contract. The potential energy of gravitational contraction heats the interior to 5 GK/430 keV and this opposes and delays the contraction. However, since no additional heat energy can be generated via new fusion reactions, the contraction rapidly accelerates into a collapse lasting only a few seconds. The central portion of the star gets crushed into either a neutron star
Neutron star
A neutron star is a type of stellar remnant that can result from the gravitational collapse of a massive star during a Type II, Type Ib or Type Ic supernova event. Such stars are composed almost entirely of neutrons, which are subatomic particles without electrical charge and with a slightly larger...
or, if the star is massive enough, a black hole
Black hole
A black hole is a region of spacetime from which nothing, not even light, can escape. The theory of general relativity predicts that a sufficiently compact mass will deform spacetime to form a black hole. Around a black hole there is a mathematically defined surface called an event horizon that...
. The outer layers of the star are blown off in an explosion known as a Type II
Type II supernova
A Type II supernova results from the rapid collapse and violent explosion of a massive star. A star must have at least 9 times, and no more than 40–50 times the mass of the Sun for this type of explosion. It is distinguished from other types of supernova by the presence of hydrogen in its spectrum...
supernova
Supernova
A supernova is a stellar explosion that is more energetic than a nova. It is pronounced with the plural supernovae or supernovas. Supernovae are extremely luminous and cause a burst of radiation that often briefly outshines an entire galaxy, before fading from view over several weeks or months...
that lasts days to months. The supernova explosion releases a large burst of neutrons, which synthesizes in about one second roughly half the elements heavier than iron, via a neutron-capture mechanism known as the r-process
R-process
The r-process is a nucleosynthesis process, likely occurring in core-collapse supernovae responsible for the creation of approximately half of the neutron-rich atomic nuclei that are heavier than iron. The process entails a succession of rapid neutron captures on seed nuclei, typically Ni-56,...
(where the “r” stands for rapid neutron capture).
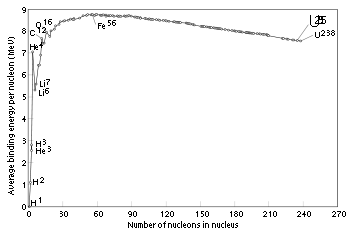
Binding energy
The graph below shows the binding energy of various elements. Increasing values of binding energy can be thought of in two ways: 1) it is the energy required to remove a nucleon from a nucleus, and 2) it is the energy released when a nucleon is added to a nucleus. As can be seen, light elements such as hydrogen release large amounts of energy (a big increase in binding energy) as nucleons are added—the process of fusion. Conversely, heavy elements such as uranium release energy when nucleons are removed—the process of nuclear fissionNuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
. In stars, rapid nucleosynthesis proceeds by adding helium nuclei (alpha particles) to heavier nuclei. Although nuclei with 58 and 62 nucleons have the very lowest binding energy, fusing a helium nucleus into nickel–56 (14 alphas) to produce the next element—zinc–60 (15 alphas)—actually requires energy rather than releases any. Accordingly, nickel–56 is the last fusion product produced in the core of a high-mass star. Decay of nickel-56 explains the large amount of iron-56 seen in metallic meteorites and the cores of rocky planets.

See also
- Stellar evolutionStellar evolutionStellar evolution is the process by which a star undergoes a sequence of radical changes during its lifetime. Depending on the mass of the star, this lifetime ranges from only a few million years to trillions of years .Stellar evolution is not studied by observing the life of a single...
- Supernova nucleosynthesisSupernova nucleosynthesisSupernova nucleosynthesis is the production of new chemical elements inside supernovae. It occurs primarily due to explosive nucleosynthesis during explosive oxygen burning and silicon burning...
- Neutron capture: p-processP-processThe term p-process is used in two ways in the scientific literature concerning the astrophysical origin of the elements . Originally it referred to a proton capture process which is the source of certain, naturally occurring, proton-rich isotopes of the elements from selenium to mercury...
, r-processR-processThe r-process is a nucleosynthesis process, likely occurring in core-collapse supernovae responsible for the creation of approximately half of the neutron-rich atomic nuclei that are heavier than iron. The process entails a succession of rapid neutron captures on seed nuclei, typically Ni-56,...
, s-processS-processThe S-process or slow-neutron-capture-process is a nucleosynthesis process that occurs at relatively low neutron density and intermediate temperature conditions in stars. Under these conditions the rate of neutron capture by atomic nuclei is slow relative to the rate of radioactive beta-minus decay...
- Neutron capture: p-process

