
Serpin
Encyclopedia
Serpins are a group of protein
s with similar structures that were first identified as a set of proteins able to inhibit
protease
s. The acronym serpin was originally coined because many serpins inhibit chymotrypsin-like serine protease
s (serine protease inhibitors).
The first members of the serpin superfamily to be extensively studied were the human plasma
proteins antithrombin
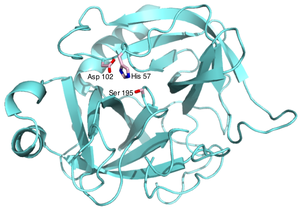
and antitrypsin, which play key roles in controlling blood coagulation
(e.g. Figure 1) and inflammation
, respectively. Initially, research focused upon their role in human disease
: antithrombin deficiency results in thrombosis
and antitrypsin deficiency causes emphysema
. In 1980 Hunt and Dayhoff made the surprising discovery that both these molecules share significant amino acid sequence similarity
to the major protein in chicken egg white
, ovalbumin
, and they proposed a new protein superfamily. Over 1000 serpins have now been identified, these include 36 human proteins, as well as molecules in plants, fungi, bacteria
, archaea
and certain viruses
. Serpins are thus the largest and most diverse family of protease inhibitors.
While most serpins control proteolytic cascades, certain serpins do not inhibit enzymes, but instead perform diverse functions such as storage
(ovalbumin, in egg white), hormone carriage proteins (thyroxine-binding globulin
, cortisol-binding globulin
) and tumor suppressor gene
s (maspin
). The term serpin is used to describe these latter members as well, despite their noninhibitory function.
As serpins control processes such as coagulation and inflammation, these proteins are the target of medical research. However, serpins are also of particular interest to the structural biology
and protein folding
communities, because they undergo a unique and dramatic change in shape (or conformational change
) when they inhibit target proteases. This is unusual - most classical protease inhibitors function as simple "lock and key" molecules that bind to and block access to the protease active site (see, for example, bovine pancreatic trypsin inhibitor
). While the serpin mechanism of protease inhibition confers certain advantages, it also has drawbacks, and serpins are vulnerable to mutations that result in protein misfolding
and the formation of inactive long-chain polymers (serpinopathies). Serpin polymerisation reduces the amount of active inhibitor, as well as accumulation of serpin polymers, causing cell death and organ failure. For example, the serpin antitrypsin is primarily produced in the liver
, and antitrypsin polymerisation causes liver cirrhosis
. Understanding serpinopathies also provides insights on protein misfolding in general, a process common to many human diseases, such as Alzheimer’s and CJD
.
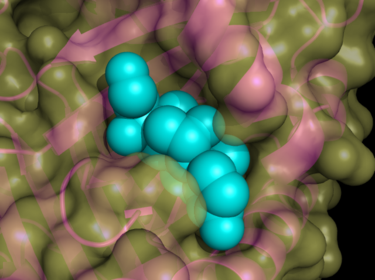
 Most inhibitory serpins target chymotrypsin
Most inhibitory serpins target chymotrypsin
-like serine protease
s (see Table 1 and Figure 2). These enzymes are defined by the presence of a nucleophilic
serine
residue in their catalytic site. Examples include thrombin
, trypsin
, and human neutrophil elastase
.
Some serpins inhibit other classes of protease
and are termed "cross-class inhibitors". A number of such serpins have been shown to target cysteine protease
s. These enzymes differ from serine protease
s in that they are defined by the presence of a nucleophilic
cysteine
residue, rather than a serine
residue, in their catalytic site. Nonetheless, the enzymatic chemistry is similar, and serpins most likely inhibit both classes of enzyme
in a similar fashion.
Examples of cross-class inhibitory serpins include squamous cell carcinoma
antigen
1 (SCCA-1) and the avian serpin myeloid and erythroid nuclear termination stage-specific protein (MENT) both inhibit papain
-like cysteine protease
s
The viral serpin crmA is a suppressor of the inflammatory response through inhibition of IL-1 and IL-18 processing by the cysteine protease caspase
-1. In eukaryotes, a plant serpin has been shown to inhibit metacaspase
s and a papain-like cysteine protease. It is presently unclear whether any mammalian serpins function to inhibit caspases in vivo.
 Approximately two-thirds of human serpins perform extracellular roles. For example, extracellular serpins regulate the proteolytic cascades central to blood clotting (antithrombin), the inflammatory response (antitrypsin, antichymotrypsin, and C1 inhibitor) and tissue remodeling (PAI-1). Non-inhibitory extracellular serpins also perform important roles. Thyroxine-binding globulin and cortisol-binding globulin transport the sterol hormones thyroxine
Approximately two-thirds of human serpins perform extracellular roles. For example, extracellular serpins regulate the proteolytic cascades central to blood clotting (antithrombin), the inflammatory response (antitrypsin, antichymotrypsin, and C1 inhibitor) and tissue remodeling (PAI-1). Non-inhibitory extracellular serpins also perform important roles. Thyroxine-binding globulin and cortisol-binding globulin transport the sterol hormones thyroxine
and cortisol
, respectively (Figure 3). The protease renin
cleaves off a ten-amino acid N-terminal peptide from angiotensinogen to produce the peptide hormone angiotensin I. Table 1 provides a brief summary of human serpin function, as well as some of the diseases that result from serpin deficiency.
The first Intracellular members of the serpin superfamily were identified in the early 1990s. As all nine serpins in Caenorhabditis elegans
lack signal sequences, they are probably intracellular. Based upon these data it seems likely that the ancestral serpin to human serpins was an intracellular molecule.
The protease targets of intracellular inhibitory serpins have been more difficult to identify. Characterization is complicated by the observation that many of these molecules appear to perform overlapping roles. Further many human serpins lack precise functional equivalents in model organisms such as the mouse. An important function of intracellular serpins may be to protect against the inappropriate activity of proteases inside the cell. For example, one of the best-characterised human intracellular serpins is SERPINB9, which inhibits the cytotoxic granule protease granzyme B. In doing so, SERPINB9 may protect against inadvertent release of granzyme B and premature or unwanted activation of cell death
pathways.
Intracellular serpins also perform roles distinct from protease inhibition. For example, maspin, a non-inhibitory serpin, is important for preventing metastasis
in breast and prostate cancers. Another example is the avian nuclear cysteine protease inhibitor MENT
, which acts as a chromatin
remodelling molecule in avian red blood cell
s.
Phylogenetic studies show that most intracellular serpins belong to a single clade (see Table 1). Exceptions include the non-inhibitory heat shock serpin HSP47, which is a chaperone essential for proper folding of collagen
, and cycles between the cis-Golgi
and the endoplasmic reticulum
.
 Structural biology
Structural biology
has played a central role in the understanding of serpin function and biology. Over eighty serpin structures, in a variety of different conformations (described below), have been determined to date. Although the function of serpins varies widely, these molecules all share a common structure (or fold).
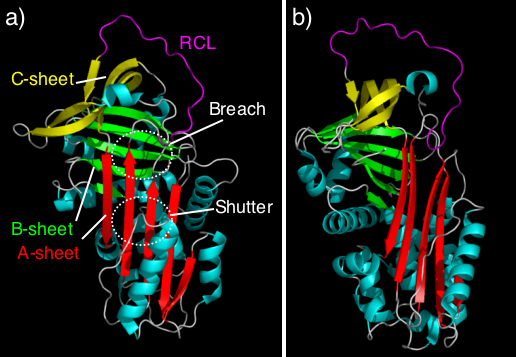
The structure of the non-inhibitory serpin ovalbumin
, and the inhibitory serpin antitrypsin, revealed the archetype native serpin fold. All typically have three β-sheets
(termed A, B and C) and eight or nine α-helices
(hA-hI) (see figure 4). Serpins also possess an exposed region termed the reactive centre loop (RCL) that, in inhibitory molecules, includes the specificity determining region and forms the initial interaction with the target protease
. In antitrypsin, the RCL is held at the top of the molecule and is not pre-inserted into the A β-sheet (figure 4, left panel). This conformation commonly exists in dynamic equilibrium
with a partially inserted native conformation seen in other inhibitory serpins (see figure 4, right panel).
When attacking a substrate
, serine proteases catalyze peptide bond cleavage in a two-step process. Initially, the catalytic serine performs a nucleophilic
attack on the peptide bond of the substrate (Figure 5). This releases the new N-terminus and forms an ester
-bond between the enzyme and the substrate. This covalent enzyme-substrate complex is called an acyl enzyme intermediate. Subsequent to this, this ester bond is hydrolysed
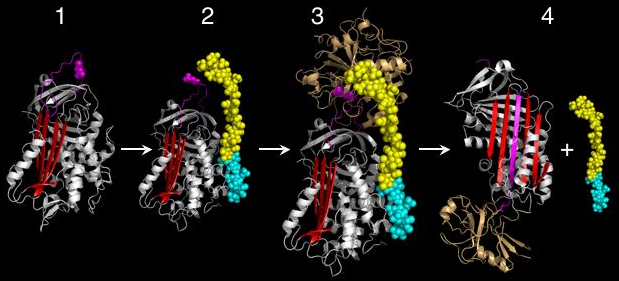
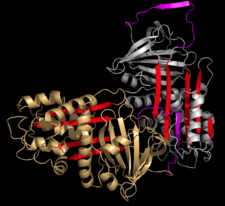
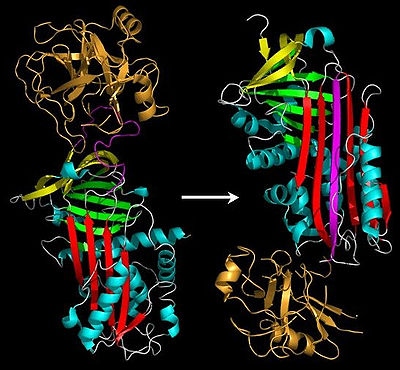
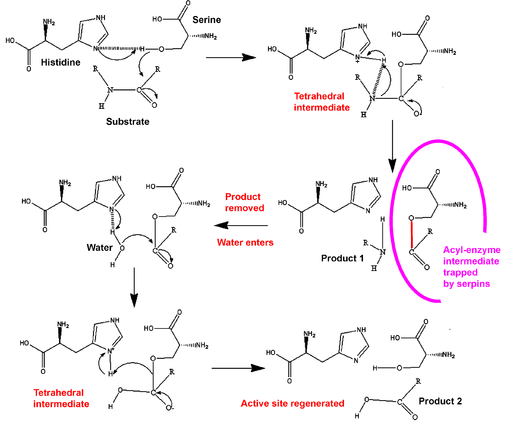
and the new C-terminus is released. The RCL of a serpin acts as a substrate for its cognate protease. However, after the RCL is cleaved, but prior to hydrolysis of the acyl-enzyme intermediate, the serpin rapidly undergoes the S-to-R transition. Since the RCL is still covalently attached to the protease via the ester bond, the S-to-R transition causes the protease to be moved from the top to the bottom of the serpin. At the same time, the protease is distorted into a conformation, where the acyl enzyme intermediate is hydrolysed extremely slowly. The protease thus remains covalently attached to the target protease and is thereby inhibited. Further, since the serpin has to be cleaved to inhibit the target protases, inhibition consumes the serpin as well. Serpins are therefore irreversible enzyme inhibitors. The serpin mechanism of inhibition is illustrated in figures 5 and 6, and several movies illustrating the serpin mechanism can be viewed at this link.
s. The X-ray crystal structures of antithrombin
, heparin cofactor II
, MENT and murine antichymotrypsin
reveal that these serpins adopt a conformation wherein the first two amino acids of the RCL are inserted into the top of the A β-sheet (see figures 4 and 7). The partially inserted conformation is important because co-factors are able to conformationally switch certain partially inserted serpins into a fully expelled form. This conformational rearrangement makes the serpin a more effective inhibitor.
The archetypal example of this situation is antithrombin, which circulates in plasma in a partially inserted relatively inactive state. The primary specificity determining residue (the P1 Arginine) points toward the body of the serpin and is unavailable to the protease (Figure 7). Upon binding a high-affinity heparin pentasaccharide sequence within long-chain heparin, antithrombin undergoes a conformational change, RCL expulsion, and exposure of the P1 Arginine. The heparin
pentasaccharide-bound form of antithrombin is, thus, a more effective inhibitor of thrombin
and factor Xa (figure 7). Furthermore, both of these coagulation proteases contain binding sites (called exosite
s) for heparin. Heparin, therefore, also acts as a template for binding of both protease and serpin, further dramatically accelerating the interaction between the two parties (Figure 7). After the initial interaction, the final serpin complex is formed and the heparin moiety is released. This interaction is physiologically important. For example, after injury to the blood vessel wall, heparin is exposed, and antithrombin is activated to control the clotting response. The understanding of the molecular basis of this interaction formed the basis of the development of Fondaparinux
, a synthetic form of Heparin pentasaccharide used as an anti-clotting drug
.
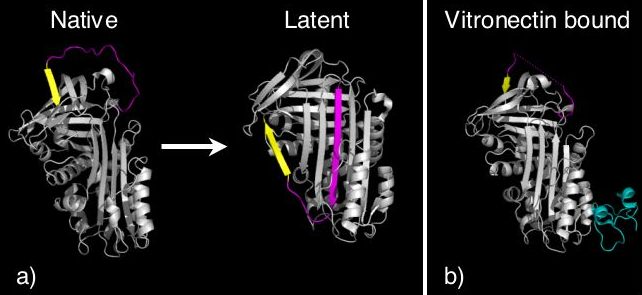
 Certain serpins spontaneously undergo the S-to-R transition as part of their function, to form a conformation termed the latent state (Figure 8). In latent serpins, the first strand of the C-sheet has to peel off to allow full RCL insertion. Latent serpins are unable to interact with proteases and are not protease inhibitors. The transition to latency represents a control mechanism for the serpin PAI-1. PAI-1 is released in the inhibitory conformation, however, undergoes conformational change to the latent state unless it is bound to the cofactor
Certain serpins spontaneously undergo the S-to-R transition as part of their function, to form a conformation termed the latent state (Figure 8). In latent serpins, the first strand of the C-sheet has to peel off to allow full RCL insertion. Latent serpins are unable to interact with proteases and are not protease inhibitors. The transition to latency represents a control mechanism for the serpin PAI-1. PAI-1 is released in the inhibitory conformation, however, undergoes conformational change to the latent state unless it is bound to the cofactor
vitronectin
. Thus PAI-1 contains an "auto-inactivation" mechanism. Similarly, antithrombin can also spontaneously convert to the latent state as part of its normal function. Finally, the N-terminus of tengpin (see pdbs 2PEE and 2PEF), a serpin from Thermoanaerobacter tengcongensis, is required to lock the molecule in the native inhibitory state. Disruption of interactions made by the N-terminal region results in spontaneous conformational change of this serpin to the latent conformation.
Recently, it has been shown that the Drosophila
serpin necrotic is taken up via the Lipophorin Receptor-1 (LpR1), which is related to the mammalian LDL receptor family. Trafficking studies reveal that following uptake by LpR1, necrotic is delivered to lysosomes where it is targeted for degradation.
has high affinity for thyroxine, whereas the cleaved (R) form has low affinity. In similar manner, native (S) Cortisol-Binding Globulin (CBG) has higher affinity for cortisol than its cleaved (R) counterpart (Figure 3). Thus, in these serpins, RCL cleavage and the S to R transition has been commandeered to allow for ligand release, rather than protease inhibition.
 Serpins are vulnerable to inactivating disease-causing mutations that result in the formation of misfolded polymers or protein aggregates
Serpins are vulnerable to inactivating disease-causing mutations that result in the formation of misfolded polymers or protein aggregates
("serpinopathies"). Well-characterised serpinopathies include alpha 1-antitrypsin deficiency
(alpha-1), which may cause familial emphysema
and sometimes liver cirrhosis
, certain familial forms of thrombosis
related to antithrombin deficiency, types 1 and 2 hereditary angioedema
(HAE) related to deficiency of C1-inhibitor
, and familial encephalopathy with neuroserpin inclusion bodies
(FENIB; a rare type of dementia
caused by neuroserpin polymerisation). Serpins thus belong to a large group of molecules such as the prion
proteins and the glutamine repeat containing proteins that cause proteopathies
or conformational diseases.
Serpin polymerisation causes disease in two ways. First, the lack of active serpin results in uncontrolled protease activity and tissue destruction; this is seen in the case of antitrypsin deficiency. Second, the polymers themselves clog up the endoplasmic reticulum
of cells that synthesize serpins, eventually resulting in cell death and tissue damage. In the case of antitrypsin deficiency, antitrypsin polymers cause the death of liver
cells, sometimes resulting in liver damage and cirrhosis
. Within the cell, serpin polymers are removed via endoplasmic reticulum associated degradation. However, the mechanism by which serpin polymers cause cell death remains to be fully understood.
Like cleaved serpins, serpin polymers are hyperstable with respect to heating, and each serpin monomer appears to have undergone the stressed to relaxed transition. Furthermore, serpin polymers are unable to inhibit target proteases, suggesting that the RCL is unavailable and inserted into the A-sheet. In the absence of definitive structural data, it was, therefore, postulated that serpins polymerise via a mechanism known as A-sheet polymerisation. In normal function the RCL inserts into the A β-sheet to form a fourth strand (figure 4). In the A-sheet polymerisation model, it was suggested that the RCL of one serpin molecule spontaneously inserted into the A-sheet of another, to form a long-chain polymer (figure 9). In effect, it was, thus, proposed that polymerization occurred as a consequence of the requirement of the serpin scaffold to accept an additional β-strand.
Serpins were one of the first families for which disease-causing mutations were directly analyzed in reference to the available crystal structures. In support of the A-sheet polymerisation model, it was noted that many serpin mutations that cause polymerisation localise to two distinct regions of the molecule (highlighted in figure 4a) termed the shutter and the breach. The shutter and the breach contain highly conserved residues, underlie the path of RCL insertion, and are proposed to be important for conformational change.
Two structures of cleaved serpin polymers have been solved; both of which reveal RCL / A-sheet sheet linkages similar to those predicted by the A sheet polymerisation mechanism. However, in direct contrast to the known properties of physiological serpin polymers, crystals of cleaved serpin A-sheet polymers readily dissociate into monomeric forms.
 A large body of data now suggest that the events associated with serpin polymerisation occur during the folding of the molecule, and that mutations that cause serpinopathies interfere with the ability of the serpin to fold to the metastable native state. In normal serpin folding, the serpin rapidly moves through a key folding intermediate to attain the native state. Many studies have shown that it is the serpin folding intermediate that has the ability to polymerise, hence it is important that this folding species rapidly moves on to adopt native state. It was shown that mutations such as the Z-antitrypsin variant (Glu 342 to Lys) somehow prevented the final stage of seprin folding and caused the accumulation of the folding intermediate. As a result, population of the folding intermediate resulted in polymer formation. Interestingly, it was noted that once folded, the Z-antitrypsin variant closely resembles wild-type material in terms of thermal stability and inhibitory activity.
A large body of data now suggest that the events associated with serpin polymerisation occur during the folding of the molecule, and that mutations that cause serpinopathies interfere with the ability of the serpin to fold to the metastable native state. In normal serpin folding, the serpin rapidly moves through a key folding intermediate to attain the native state. Many studies have shown that it is the serpin folding intermediate that has the ability to polymerise, hence it is important that this folding species rapidly moves on to adopt native state. It was shown that mutations such as the Z-antitrypsin variant (Glu 342 to Lys) somehow prevented the final stage of seprin folding and caused the accumulation of the folding intermediate. As a result, population of the folding intermediate resulted in polymer formation. Interestingly, it was noted that once folded, the Z-antitrypsin variant closely resembles wild-type material in terms of thermal stability and inhibitory activity.
Together, these data have presented an important challenge to the A-sheet model for serpin polymerisation. On the one hand, the idea that serpin polymer formation essentially takes advantage of the serpin mechanism of conformational change is an attractive one. On the other, the biophyiscal data in particular suggest that it is a folding intermediate (rather than the native form) that polymerises, and it is clear that this intermediate must have different structural properties to the native, folded state.
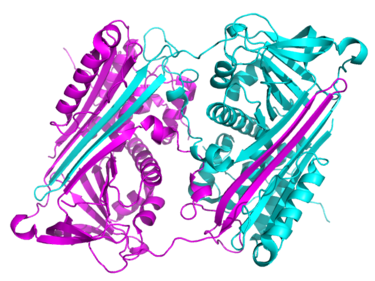
In 2008, a key serpin crystal structure was determined that strongly suggests that physiological serpin polymers do not form via the A-sheet mechanism and instead form via a more extensive domain swapping
event. The first such structure solved was of an antithrombin dimer (figure 10), and revealed that both strands s5A and the RCL can be incorporated into the A-sheet of another serpin molecule. This structure can readily be adapted to form long chain polymers. In 2011, the structure of a domain swapped antitrypsin trimer revealed that in polymers of this serpin the RCL is inserted, and that the C-terminal region of the molecule (comprising strands s1C, s4B and s5B) formed the domain swap (figure 11). In support of the physiological relevance of the latter structure, it was shown that antitrypsin polymers formed via a C-terminal domain swap were recognised by a monocloncal antibody specific for pathogenic antitrypsin polymers.
The new "domain swapped" model for serpin polymerisation begins to reconcile the available biophysical and biochemical data. Together, these data suggest that domain swapping events occur when mutations or environmental factors somehow interfere with the final stages of serpin folding to the native state. These data also reveal that different serpins can apparently polymerise via different types of domain swaps. Finally, while these data shed light on the final polymeric form, it is important to note that the precise toxic species of intermediate and / or polymer that causes cell death in, for example, antitrypsin deficiency, remains to be identified.
 Certain pathogenic mutations in serpins can promote inappropriate transition to the monomeric latent state (see figure 8a for the structure of the latent state). This causes disease because it reduces the amount of active inhibitory serpin. For example, the disease-linked antithrombin variants wibble and wobble, both promote formation of the latent state.
Certain pathogenic mutations in serpins can promote inappropriate transition to the monomeric latent state (see figure 8a for the structure of the latent state). This causes disease because it reduces the amount of active inhibitory serpin. For example, the disease-linked antithrombin variants wibble and wobble, both promote formation of the latent state.
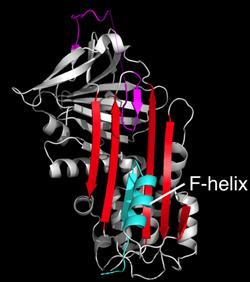
It is also worth highlighting a structure of a disease-linked human antichymotrypsin variant that further demonstrates the extraordinary flexibility of the serpin scaffold. The structure of antichymotrypsin (Leucine 55 to Proline) revealed a novel "δ" conformation that may represent an intermediate between the native and latent state (Figure 12). In the delta conformation, four residues of the RCL are inserted into the top of β-sheet A. The bottom half of the sheet is filled as a result of one of the α-helices (the F-helix) partially switching to a β-strand conformation, completing the β-sheet hydrogen bonding. It is unclear whether other serpins can adopt this conformer, and whether this conformation has a functional role. However, it is speculated that the δ-conformation may be adopted by Thyroxine-binding globulin during thyroxine release.
It is rare that single amino acid changes in the RCL of a serpin alters the specificity of the inhibitor and allow it to target the wrong protease. For example, the Antitrypsin-Pittsburgh mutation (methionine
358 to arginine
) allowed the serpin to inhibit thrombin
, thus causing a bleeding
disorder.
Serpins are suicide inhibitors, the RCL acting as a "bait." Certain disease-linked mutations in the RCL of human serpins permit true substrate-like behaviour and cleavage without complex formation. Such variants are speculated to affect the rate or the extent of RCL insertion into the A-sheet. These mutations, in effect, result in serpin deficiency through a failure to properly control the target protease.
Several non-inhibitory serpins play key roles in important human diseases. For example, maspin functions as a tumour suppressor in breast and prostate cancer. The mechanism of maspin function remains to be fully understood. Murine knockouts
of maspin are lethal; these data suggest that maspin plays a key role in development.
organisms, but have since been found in a number of bacteria
and archaea
. It remains unclear whether these prokaryote genes are the descendants of an ancestral prokaryotic serpin or the product of lateral gene transfer (genetic transfer between organisms not by evolutionary descent). Rawlings et al. showed that serpins are the most widely distributed and largest family of protease inhibitors.
The human genome encodes 16 serpin clades, termed serpinA through to serpinP, encoding 29 inhibitory and 7 non-inhibitory serpin proteins (see Law et al. (2006) for a recent review). The proteins are named serpinXY where X is the clade of the protein and Y the number of the protein within that clade. Table 1 lists each human serpin, together with brief notes in regards to each molecules function and the consequence (where known) of dysfunction or deficiency.
refers to members of the serpin A clade that are encoded by the SERPINA14 gene. Uterine serpins are produced by the uterine endometrium of a restricted group of mammals in the Laurasiatheria clade under the influence of progesterone or estrogen. They are probably not functional proteinase inhibitors and may function during pregnancy to inhibit maternal immune responses against the conceptus or to participate in transplacental transport.
genome contains 29 serpin encoding genes. Amino acid sequence analysis has placed 14 of these serpins in serpin clade Q and 3 in serpin clade K with the remaining 12 serpins classified as orphan serpins not belonging to any clade. The clade classification system is difficult to use for Drosophila serpins and instead a nomenclature system has been adopted that is based on the position of Drosphila serpin genes on the Drosophila chromosome
s. 13 of the Drosophila serpins occur as isolated genes in the genome (including Serpin-27A, see below), with the remaining 16 organised into three gene clusters that occur at chromosome positions 28D (2 serpins), 42D (5 serpins), 43A (4 serpins), 77B (3 serpins) and 88E (2 serpins).
Studies on Drosophila serpins reveal that Serpin-27A inhibits the Easter protease (the final protease in the Nudel, Gastrulation Defective, Snake and Easter proteolytic cascade) and thus controls dorsoventral patterning. Easter functions to cleave Spätzle (a chemokine-type ligand), which results in Toll
mediated signaling. In addition to its central role in embryonic patterning, Toll signaling is also important for the innate immune response in insects. Accordingly, serpin-27A additionally functions to control the insect immune response. Interestingly, in Tenebrio molitor (a large beetle), a protein (SPN93) comprising two discrete tandem serpin domains functions to regulate the toll proteolytic cascade.
contains nine serpins, however, only five of these molecules appear to function as protease inhibitors. One of these serpins, SRP-6, has been shown to perform a protective function and guard against stress induced calpain-associated lysosomal disruption. Further SRP-6 functions to inhibit lysosomal cysteine proteases released after lysosomal rupture. Accordingly, worms lacking SRP-6 are sensitive to stress. Most notably, SRP-6 knockout worms die when placed in water (the hypo-osmotic stress lethal phenotype or Osl). Based on these data it is suggested that lysosomes play a general and controllable role in determining cell fate.
The MEROPS database identifies 18 serpin family members in the Arabidopsis thaliana
genome, but only about eight of these are full-length serpin sequences. Plant serpins are potent inhibitors of mammalian chymotrypsin-like serine proteases in vitro, the most well-studied example being barley serpin Zx (BSZx), which is able to inhibit trypsin, chymotrypsin as well as several blood coagulation factors. However, close relatives of chymotrypsin-like serine proteases are absent in plants. Interestingly, the RCL of several serpins from wheat grain and rye contain poly-Q repeat sequences similar to those present in the prolamin storage proteins of the endosperm. It has therefore been suggested that plant serpins may function to inhibit proteases from insects or microbes that cleave grain storage proteins. In support of this hypothesis, specific plant serpins have been identified in the phloem sap of pumpkin (CmPS-1) and cucumber plants. However, while an inverse correlation between up-regulation of CmPS-1 expression and aphid survival was observed, in vitro feeding experiments revealed that recombinant CmPS-1 did not appear to affect insect survival.
Alternative roles and protease targets for plant serpins have been proposed. It has been shown that Arabidopsis AtSerpin1 (At1g47710; 3LE2) inhibits metacaspase
-like proteases in vitro. More recently the major in vivo protease target for AtSerpin1 was identified as the papain-like cysteine protease RESPONSIVE TO DESICCATION-21 (RD21). In the same study the X-ray crystal structure of the native, stressed form of AtSerpin1 was shown to have plant-specific features. Two other Arabidopsis serpins, AtSRP2 (At2g14540) and AtSRP3 (At1g64030) appear to be involved in responses to DNA damage caused by plant exposure to methane methylsulfonate (MMS).
sp. strain E2. Piromyces is an anaerobic fungus found in the gut of ruminants and is important for digesting plant material. Celpin is predicted to be an inhibitory molecule and contains two N-terminal dockerin domains in addition to the serpin domain. Dockerins are commonly found in proteins that localise to the fungal cellulosome, a large extracellular mulitprotein complex that breaks down cellulose. It is therefore suggested that celpin protects the cellulosome against plant proteases. Interestingly certain bacterial serpins also localize to the cellulosome.
thermocellum serpin localises to the cellulosome. It is suggested that the role of cellulosome-associated serpins may be to prevent unwanted protease activity against the cellulosome.
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s with similar structures that were first identified as a set of proteins able to inhibit
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
s. The acronym serpin was originally coined because many serpins inhibit chymotrypsin-like serine protease
Serine protease
Serine proteases are enzymes that cleave peptide bonds in proteins, in which serine serves as the nucleophilic amino acid at the active site.They are found ubiquitously in both eukaryotes and prokaryotes...
s (serine protease inhibitors).
The first members of the serpin superfamily to be extensively studied were the human plasma
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
proteins antithrombin
Antithrombin
Antithrombin is a small protein molecule that inactivates several enzymes of the coagulation system. Antithrombin is a glycoprotein produced by the liver and consists of 432 amino acids. It contains three disulfide bonds and a total of four possible glycosylation sites...
and antitrypsin, which play key roles in controlling blood coagulation
Coagulation
Coagulation is a complex process by which blood forms clots. It is an important part of hemostasis, the cessation of blood loss from a damaged vessel, wherein a damaged blood vessel wall is covered by a platelet and fibrin-containing clot to stop bleeding and begin repair of the damaged vessel...
(e.g. Figure 1) and inflammation
Inflammation
Inflammation is part of the complex biological response of vascular tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. Inflammation is a protective attempt by the organism to remove the injurious stimuli and to initiate the healing process...
, respectively. Initially, research focused upon their role in human disease
Disease
A disease is an abnormal condition affecting the body of an organism. It is often construed to be a medical condition associated with specific symptoms and signs. It may be caused by external factors, such as infectious disease, or it may be caused by internal dysfunctions, such as autoimmune...
: antithrombin deficiency results in thrombosis
Thrombosis
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets and fibrin to form a blood clot to prevent blood loss...
and antitrypsin deficiency causes emphysema
Emphysema
Emphysema is a long-term, progressive disease of the lungs that primarily causes shortness of breath. In people with emphysema, the tissues necessary to support the physical shape and function of the lungs are destroyed. It is included in a group of diseases called chronic obstructive pulmonary...
. In 1980 Hunt and Dayhoff made the surprising discovery that both these molecules share significant amino acid sequence similarity
Sequence alignment
In bioinformatics, a sequence alignment is a way of arranging the sequences of DNA, RNA, or protein to identify regions of similarity that may be a consequence of functional, structural, or evolutionary relationships between the sequences. Aligned sequences of nucleotide or amino acid residues are...
to the major protein in chicken egg white
Egg white
Egg white is the common name for the clear liquid contained within an egg. In chickens it is formed from the layers of secretions of the anterior section of the hen's oviduct during the passage of the egg. It forms around either fertilized or unfertilized egg yolks...
, ovalbumin
Ovalbumin
Ovalbumin is the main protein found in egg white, making up 60-65% of the total protein. Ovalbumin displays sequence and three-dimensional homology to the serpin superfamily, but unlike most serpins it is not a serine protease inhibitor...
, and they proposed a new protein superfamily. Over 1000 serpins have now been identified, these include 36 human proteins, as well as molecules in plants, fungi, bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
, archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
and certain viruses
Poxviridae
Poxviruses are viruses that can, as a family, infect both vertebrate and invertebrate animals.Four genera of poxviruses may infect humans: orthopox, parapox, yatapox, molluscipox....
. Serpins are thus the largest and most diverse family of protease inhibitors.
While most serpins control proteolytic cascades, certain serpins do not inhibit enzymes, but instead perform diverse functions such as storage
Storage protein
Storage proteins are biological reserves of metal ions and amino acids, used by organisms. They are found in plant seeds, egg whites, and milk....
(ovalbumin, in egg white), hormone carriage proteins (thyroxine-binding globulin
Thyroxine-binding globulin
Thyroxine-binding globulin binds thyroid hormone in circulation. It is one of three proteins responsible for carrying the thyroid hormones thyroxine and 3,5,3’-triiodothyronine in the bloodstream. Of these three proteins, TBG has the highest affinity for T4 and T3, but is present in the lowest...
, cortisol-binding globulin
Transcortin
Transcortin, also corticosteroid-binding globulin or CBG, is officially called serpin peptidase inhibitor, clade A , member 6.It is an alpha-globulin.-Binding:...
) and tumor suppressor gene
Tumor suppressor gene
A tumor suppressor gene, or anti-oncogene, is a gene that protects a cell from one step on the path to cancer. When this gene is mutated to cause a loss or reduction in its function, the cell can progress to cancer, usually in combination with other genetic changes.-Two-hit hypothesis:Unlike...
s (maspin
Maspin
Maspin is a serpin and tumor suppressor gene.It has been associated with prostate cancer and breast cancer.It has also been associated with tumors of the pancreas.It was identified in 1994.-Further reading:-External links:...
). The term serpin is used to describe these latter members as well, despite their noninhibitory function.
As serpins control processes such as coagulation and inflammation, these proteins are the target of medical research. However, serpins are also of particular interest to the structural biology
Structural biology
Structural biology is a branch of molecular biology, biochemistry, and biophysics concerned with the molecular structure of biological macromolecules, especially proteins and nucleic acids, how they acquire the structures they have, and how alterations in their structures affect their function...
and protein folding
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
communities, because they undergo a unique and dramatic change in shape (or conformational change
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
) when they inhibit target proteases. This is unusual - most classical protease inhibitors function as simple "lock and key" molecules that bind to and block access to the protease active site (see, for example, bovine pancreatic trypsin inhibitor
Aprotinin
The drug aprotinin , is the bovine version of the small protein basic pancreatic trypsin inhibitor, or BPTI, which inhibits trypsin and related proteolytic enzymes. Under the trade name Trasylol, aprotinin was used as a medication administered by injection to reduce bleeding during complex surgery,...
). While the serpin mechanism of protease inhibition confers certain advantages, it also has drawbacks, and serpins are vulnerable to mutations that result in protein misfolding
Proteopathy
In medicine, proteopathy refers to a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body...
and the formation of inactive long-chain polymers (serpinopathies). Serpin polymerisation reduces the amount of active inhibitor, as well as accumulation of serpin polymers, causing cell death and organ failure. For example, the serpin antitrypsin is primarily produced in the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
, and antitrypsin polymerisation causes liver cirrhosis
Cirrhosis
Cirrhosis is a consequence of chronic liver disease characterized by replacement of liver tissue by fibrosis, scar tissue and regenerative nodules , leading to loss of liver function...
. Understanding serpinopathies also provides insights on protein misfolding in general, a process common to many human diseases, such as Alzheimer’s and CJD
CJD
CJD can mean:*Creutzfeldt-Jakob disease, a rare disease of the brain caused by prions, related to bovine spongiform encephalopathy*Chronological Julian Day, an alternate way of expressing the Julian Date...
.
Cross-class inhibitors

Chymotrypsin
Chymotrypsin is a digestive enzyme that can perform proteolysis. Chymotrypsin preferentially cleaves peptide amide bonds where the carboxyl side of the amide bond is a tyrosine, tryptophan, or phenylalanine. These amino acids contain an aromatic ring in their sidechain that fits into a...
-like serine protease
Serine protease
Serine proteases are enzymes that cleave peptide bonds in proteins, in which serine serves as the nucleophilic amino acid at the active site.They are found ubiquitously in both eukaryotes and prokaryotes...
s (see Table 1 and Figure 2). These enzymes are defined by the presence of a nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
residue in their catalytic site. Examples include thrombin
Thrombin
Thrombin is a "trypsin-like" serine protease protein that in humans is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the first step of the coagulation cascade, which ultimately results in the stemming of blood loss...
, trypsin
Trypsin
Trypsin is a serine protease found in the digestive system of many vertebrates, where it hydrolyses proteins. Trypsin is produced in the pancreas as the inactive proenzyme trypsinogen. Trypsin cleaves peptide chains mainly at the carboxyl side of the amino acids lysine or arginine, except when...
, and human neutrophil elastase
Human neutrophil elastase
Neutrophil elastase also known as ELA2 is a serine proteinase in the same family as chymotrypsin and has broad substrate specificity...
.
Some serpins inhibit other classes of protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
and are termed "cross-class inhibitors". A number of such serpins have been shown to target cysteine protease
Cysteine protease
Proteases are enzymes that degrade polypeptides. Cysteine proteases have a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic dyad. The first step is deprotonation of a thiol in the enzyme's active site by an adjacent amino acid with a basic side chain, usually a...
s. These enzymes differ from serine protease
Serine protease
Serine proteases are enzymes that cleave peptide bonds in proteins, in which serine serves as the nucleophilic amino acid at the active site.They are found ubiquitously in both eukaryotes and prokaryotes...
s in that they are defined by the presence of a nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residue, rather than a serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
residue, in their catalytic site. Nonetheless, the enzymatic chemistry is similar, and serpins most likely inhibit both classes of enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
in a similar fashion.
Examples of cross-class inhibitory serpins include squamous cell carcinoma
Squamous cell carcinoma
Squamous cell carcinoma , occasionally rendered as "squamous-cell carcinoma", is a histologically distinct form of cancer. It arises from the uncontrolled multiplication of malignant cells deriving from epithelium, or showing particular cytological or tissue architectural characteristics of...
antigen
Antigen
An antigen is a foreign molecule that, when introduced into the body, triggers the production of an antibody by the immune system. The immune system will then kill or neutralize the antigen that is recognized as a foreign and potentially harmful invader. These invaders can be molecules such as...
1 (SCCA-1) and the avian serpin myeloid and erythroid nuclear termination stage-specific protein (MENT) both inhibit papain
Papain
Papain, also known as papaya proteinase I, is a cysteine protease enzyme present in papaya and mountain papaya .-Papain family:...
-like cysteine protease
Cysteine protease
Proteases are enzymes that degrade polypeptides. Cysteine proteases have a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic dyad. The first step is deprotonation of a thiol in the enzyme's active site by an adjacent amino acid with a basic side chain, usually a...
s
The viral serpin crmA is a suppressor of the inflammatory response through inhibition of IL-1 and IL-18 processing by the cysteine protease caspase
Caspase
Caspases, or cysteine-aspartic proteases or cysteine-dependent aspartate-directed proteases are a family of cysteine proteases that play essential roles in apoptosis , necrosis, and inflammation....
-1. In eukaryotes, a plant serpin has been shown to inhibit metacaspase
Metacaspase
Metacaspases are related to caspases and paracaspase. The metacaspases are Arginine/Lysine-specific, in contrast to caspases, which are Aspartate-specific...
s and a papain-like cysteine protease. It is presently unclear whether any mammalian serpins function to inhibit caspases in vivo.
Localization and roles

Thyroxine
Thyroxine, or 3,5,3',5'-tetraiodothyronine , a form of thyroid hormones, is the major hormone secreted by the follicular cells of the thyroid gland.-Synthesis and regulation:...
and cortisol
Cortisol
Cortisol is a steroid hormone, more specifically a glucocorticoid, produced by the adrenal gland. It is released in response to stress and a low level of blood glucocorticoids. Its primary functions are to increase blood sugar through gluconeogenesis; suppress the immune system; and aid in fat,...
, respectively (Figure 3). The protease renin
Renin
Renin , also known as an angiotensinogenase, is an enzyme that participates in the body's renin-angiotensin system -- also known as the Renin-Angiotensin-Aldosterone Axis -- that mediates extracellular volume , and arterial vasoconstriction...
cleaves off a ten-amino acid N-terminal peptide from angiotensinogen to produce the peptide hormone angiotensin I. Table 1 provides a brief summary of human serpin function, as well as some of the diseases that result from serpin deficiency.
The first Intracellular members of the serpin superfamily were identified in the early 1990s. As all nine serpins in Caenorhabditis elegans
Caenorhabditis elegans
Caenorhabditis elegans is a free-living, transparent nematode , about 1 mm in length, which lives in temperate soil environments. Research into the molecular and developmental biology of C. elegans was begun in 1974 by Sydney Brenner and it has since been used extensively as a model...
lack signal sequences, they are probably intracellular. Based upon these data it seems likely that the ancestral serpin to human serpins was an intracellular molecule.
The protease targets of intracellular inhibitory serpins have been more difficult to identify. Characterization is complicated by the observation that many of these molecules appear to perform overlapping roles. Further many human serpins lack precise functional equivalents in model organisms such as the mouse. An important function of intracellular serpins may be to protect against the inappropriate activity of proteases inside the cell. For example, one of the best-characterised human intracellular serpins is SERPINB9, which inhibits the cytotoxic granule protease granzyme B. In doing so, SERPINB9 may protect against inadvertent release of granzyme B and premature or unwanted activation of cell death
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
pathways.
Intracellular serpins also perform roles distinct from protease inhibition. For example, maspin, a non-inhibitory serpin, is important for preventing metastasis
Metastasis
Metastasis, or metastatic disease , is the spread of a disease from one organ or part to another non-adjacent organ or part. It was previously thought that only malignant tumor cells and infections have the capacity to metastasize; however, this is being reconsidered due to new research...
in breast and prostate cancers. Another example is the avian nuclear cysteine protease inhibitor MENT
Myeloid and erythroid nuclear termination stage specific protein
Myeloid and erythroid nuclear termination stage-specific protein is a member of the serpin family of protease inhibitors, and participates in DNA and chromatin condensation. Alongside its ability to condense chromatin, MENT is also an effective inhibitor of the proteases cathepsin K, cathepsin L,...
, which acts as a chromatin
Chromatin
Chromatin is the combination of DNA and proteins that make up the contents of the nucleus of a cell. The primary functions of chromatin are; to package DNA into a smaller volume to fit in the cell, to strengthen the DNA to allow mitosis and meiosis and prevent DNA damage, and to control gene...
remodelling molecule in avian red blood cell
Red blood cell
Red blood cells are the most common type of blood cell and the vertebrate organism's principal means of delivering oxygen to the body tissues via the blood flow through the circulatory system...
s.
Phylogenetic studies show that most intracellular serpins belong to a single clade (see Table 1). Exceptions include the non-inhibitory heat shock serpin HSP47, which is a chaperone essential for proper folding of collagen
Collagen
Collagen is a group of naturally occurring proteins found in animals, especially in the flesh and connective tissues of mammals. It is the main component of connective tissue, and is the most abundant protein in mammals, making up about 25% to 35% of the whole-body protein content...
, and cycles between the cis-Golgi
Golgi apparatus
The Golgi apparatus is an organelle found in most eukaryotic cells. It was identified in 1898 by the Italian physician Camillo Golgi, after whom the Golgi apparatus is named....
and the endoplasmic reticulum
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
.
Structure

X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
has played a central role in the understanding of serpin function and biology. Over eighty serpin structures, in a variety of different conformations (described below), have been determined to date. Although the function of serpins varies widely, these molecules all share a common structure (or fold).
The structure of the non-inhibitory serpin ovalbumin
Ovalbumin
Ovalbumin is the main protein found in egg white, making up 60-65% of the total protein. Ovalbumin displays sequence and three-dimensional homology to the serpin superfamily, but unlike most serpins it is not a serine protease inhibitor...
, and the inhibitory serpin antitrypsin, revealed the archetype native serpin fold. All typically have three β-sheets
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
(termed A, B and C) and eight or nine α-helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
(hA-hI) (see figure 4). Serpins also possess an exposed region termed the reactive centre loop (RCL) that, in inhibitory molecules, includes the specificity determining region and forms the initial interaction with the target protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
. In antitrypsin, the RCL is held at the top of the molecule and is not pre-inserted into the A β-sheet (figure 4, left panel). This conformation commonly exists in dynamic equilibrium
Dynamic equilibrium
A dynamic equilibrium exists once a reversible reaction ceases to change its ratio of reactants/products, but substances move between the chemicals at an equal rate, meaning there is no net change. It is a particular example of a system in a steady state...
with a partially inserted native conformation seen in other inhibitory serpins (see figure 4, right panel).
Conformational change and inhibitory mechanism
Early studies on serpins revealed that the mechanism by which these molecules inhibit target proteases appeared distinct from the lock-and-key-type mechanism utilised by small protease inhibitors such as the Kunitz-type inhibitors (e.g. basic pancreatic trypsin inhibitor). Indeed, serpins form covalent complexes with target proteases. Structural studies on serpins also revealed that inhibitory members of the family undergo an unusual conformational change, termed the Stressed to Relaxed (S to R) transition. During this structural transition the RCL inserts into β-sheet A (in red in figure 4 and 5) and forms an extra (fourth) β-strand. The serpin conformational change is key to the mechanism of inhibition of target proteases.When attacking a substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
, serine proteases catalyze peptide bond cleavage in a two-step process. Initially, the catalytic serine performs a nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
attack on the peptide bond of the substrate (Figure 5). This releases the new N-terminus and forms an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
-bond between the enzyme and the substrate. This covalent enzyme-substrate complex is called an acyl enzyme intermediate. Subsequent to this, this ester bond is hydrolysed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
and the new C-terminus is released. The RCL of a serpin acts as a substrate for its cognate protease. However, after the RCL is cleaved, but prior to hydrolysis of the acyl-enzyme intermediate, the serpin rapidly undergoes the S-to-R transition. Since the RCL is still covalently attached to the protease via the ester bond, the S-to-R transition causes the protease to be moved from the top to the bottom of the serpin. At the same time, the protease is distorted into a conformation, where the acyl enzyme intermediate is hydrolysed extremely slowly. The protease thus remains covalently attached to the target protease and is thereby inhibited. Further, since the serpin has to be cleaved to inhibit the target protases, inhibition consumes the serpin as well. Serpins are therefore irreversible enzyme inhibitors. The serpin mechanism of inhibition is illustrated in figures 5 and 6, and several movies illustrating the serpin mechanism can be viewed at this link.
 |
 |
Conformational modulation of serpin activity
The conformational mobility of serpins provides a key advantage over static lock-and-key protease inhibitors. In particular, the function of inhibitory serpins can be readily controlled by specific cofactorCofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
s. The X-ray crystal structures of antithrombin
Antithrombin
Antithrombin is a small protein molecule that inactivates several enzymes of the coagulation system. Antithrombin is a glycoprotein produced by the liver and consists of 432 amino acids. It contains three disulfide bonds and a total of four possible glycosylation sites...
, heparin cofactor II
Heparin cofactor II
Heparin cofactor II, a protein encoded by the SERPIND1 gene, is a coagulation factor that inhibits IIa, and is a cofactor for heparin and dermatan sulfate...
, MENT and murine antichymotrypsin
Alpha 1-antichymotrypsin
Alpha 1-antichymotrypsin is an alpha globulin glycoprotein that is a member of the serpin superfamily.It inhibits the activity of certain enzymes called proteases, such as cathepsin G that is found in neutrophils, and chymases found in mast cells, by cleaving them into a different shape or...
reveal that these serpins adopt a conformation wherein the first two amino acids of the RCL are inserted into the top of the A β-sheet (see figures 4 and 7). The partially inserted conformation is important because co-factors are able to conformationally switch certain partially inserted serpins into a fully expelled form. This conformational rearrangement makes the serpin a more effective inhibitor.
The archetypal example of this situation is antithrombin, which circulates in plasma in a partially inserted relatively inactive state. The primary specificity determining residue (the P1 Arginine) points toward the body of the serpin and is unavailable to the protease (Figure 7). Upon binding a high-affinity heparin pentasaccharide sequence within long-chain heparin, antithrombin undergoes a conformational change, RCL expulsion, and exposure of the P1 Arginine. The heparin
Heparin
Heparin , also known as unfractionated heparin, a highly sulfated glycosaminoglycan, is widely used as an injectable anticoagulant, and has the highest negative charge density of any known biological molecule...
pentasaccharide-bound form of antithrombin is, thus, a more effective inhibitor of thrombin
Thrombin
Thrombin is a "trypsin-like" serine protease protein that in humans is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the first step of the coagulation cascade, which ultimately results in the stemming of blood loss...
and factor Xa (figure 7). Furthermore, both of these coagulation proteases contain binding sites (called exosite
Exosite
An exosite is a secondary binding site, remote from the active site, on an enzyme or other protein.This is similar to allosteric sites, but differs in the fact that, in order for an enzyme to be active, its exosite typically must be occupied. Exosites have recently become a hot topic in biomedical...
s) for heparin. Heparin, therefore, also acts as a template for binding of both protease and serpin, further dramatically accelerating the interaction between the two parties (Figure 7). After the initial interaction, the final serpin complex is formed and the heparin moiety is released. This interaction is physiologically important. For example, after injury to the blood vessel wall, heparin is exposed, and antithrombin is activated to control the clotting response. The understanding of the molecular basis of this interaction formed the basis of the development of Fondaparinux
Fondaparinux
Fondaparinux is an anticoagulant medication chemically related to low molecular weight heparins. It is marketed by GlaxoSmithKline.-Structure and mechanism:Fondaparinux is a synthetic pentasaccharide Factor Xa inhibitor...
, a synthetic form of Heparin pentasaccharide used as an anti-clotting drug
Anticoagulant
An anticoagulant is a substance that prevents coagulation of blood. A group of pharmaceuticals called anticoagulants can be used in vivo as a medication for thrombotic disorders. Some anticoagulants are used in medical equipment, such as test tubes, blood transfusion bags, and renal dialysis...
.

Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
vitronectin
Vitronectin
Vitronectin also known as VTN is a protein that in humans is encoded by the VTN gene.The protein encoded by this gene is a member of the pexin family...
. Thus PAI-1 contains an "auto-inactivation" mechanism. Similarly, antithrombin can also spontaneously convert to the latent state as part of its normal function. Finally, the N-terminus of tengpin (see pdbs 2PEE and 2PEF), a serpin from Thermoanaerobacter tengcongensis, is required to lock the molecule in the native inhibitory state. Disruption of interactions made by the N-terminal region results in spontaneous conformational change of this serpin to the latent conformation.

Serpin receptor interactions
In humans, extracellular serpin-enzyme complexes are rapidly cleared from circulation. In mammals, one mechanism by which this occurs is via the low-density lipoprotein receptor-related protein (LRP receptor), which binds to inhibitory complexes made by antithrombin, PA1-1, and neuroserpin, causing uptake and subsequent signaling events. Thus, as a consequence of the conformational change during serpin-enzyme complex formation, serpins may act as signaling molecules that alert cells to the presence of protease activity. The fate of intracellular serpin-enzyme complexes remains to be characterised.Recently, it has been shown that the Drosophila
Drosophila
Drosophila is a genus of small flies, belonging to the family Drosophilidae, whose members are often called "fruit flies" or more appropriately pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species to linger around overripe or rotting fruit...
serpin necrotic is taken up via the Lipophorin Receptor-1 (LpR1), which is related to the mammalian LDL receptor family. Trafficking studies reveal that following uptake by LpR1, necrotic is delivered to lysosomes where it is targeted for degradation.
Conformational change and non-inhibitory function
Certain non-inhibitory serpins also use the serpin conformational change as part of their function. For example, the native (S) form of thyroxine-binding globulinThyroxine-binding globulin
Thyroxine-binding globulin binds thyroid hormone in circulation. It is one of three proteins responsible for carrying the thyroid hormones thyroxine and 3,5,3’-triiodothyronine in the bloodstream. Of these three proteins, TBG has the highest affinity for T4 and T3, but is present in the lowest...
has high affinity for thyroxine, whereas the cleaved (R) form has low affinity. In similar manner, native (S) Cortisol-Binding Globulin (CBG) has higher affinity for cortisol than its cleaved (R) counterpart (Figure 3). Thus, in these serpins, RCL cleavage and the S to R transition has been commandeered to allow for ligand release, rather than protease inhibition.
Serpins, serpinopathies and human disease

Protein aggregation
Protein aggregation is the aggregation of mis-folded proteins, and is thought to be responsible for many degenerative diseases, such as Alzheimer's. It has also been implicated in CAG repeat diseases....
("serpinopathies"). Well-characterised serpinopathies include alpha 1-antitrypsin deficiency
Alpha 1-antitrypsin deficiency
Alpha 1-antitrypsin deficiency is an autosomal recessive genetic disorder caused by defective production of alpha 1-antitrypsin , leading to decreased A1AT activity in the blood and lungs, and deposition of excessive abnormal A1AT protein in liver cells...
(alpha-1), which may cause familial emphysema
Emphysema
Emphysema is a long-term, progressive disease of the lungs that primarily causes shortness of breath. In people with emphysema, the tissues necessary to support the physical shape and function of the lungs are destroyed. It is included in a group of diseases called chronic obstructive pulmonary...
and sometimes liver cirrhosis
Cirrhosis
Cirrhosis is a consequence of chronic liver disease characterized by replacement of liver tissue by fibrosis, scar tissue and regenerative nodules , leading to loss of liver function...
, certain familial forms of thrombosis
Thrombosis
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets and fibrin to form a blood clot to prevent blood loss...
related to antithrombin deficiency, types 1 and 2 hereditary angioedema
Angioedema
Angioedema or Quincke's edema is the rapid swelling of the dermis, subcutaneous tissue, mucosa and submucosal tissues. It is very similar to urticaria, but urticaria, commonly known as hives, occurs in the upper dermis...
(HAE) related to deficiency of C1-inhibitor
C1-inhibitor
C1-inhibitor is a protease inhibitor belonging to the serpin superfamily. Its main function is the inhibition of the complement system to prevent spontaneous activation. C1-inhibitor is an acute-phase protein that circulates in blood at levels of around 0.25 g/L. The levels rise ~2-fold during...
, and familial encephalopathy with neuroserpin inclusion bodies
Familial encephalopathy with neuroserpin inclusion bodies
Familial encephalopathy with neuroserpin inclusion bodies is a progressive disorder of the nervous system that is characterized by a loss of intellectual functioning and seizures. At first, affected individuals may have difficulty sustaining attention and concentrating. Their judgment, insight,...
(FENIB; a rare type of dementia
Dementia
Dementia is a serious loss of cognitive ability in a previously unimpaired person, beyond what might be expected from normal aging...
caused by neuroserpin polymerisation). Serpins thus belong to a large group of molecules such as the prion
Prion
A prion is an infectious agent composed of protein in a misfolded form. This is in contrast to all other known infectious agents which must contain nucleic acids . The word prion, coined in 1982 by Stanley B. Prusiner, is a portmanteau derived from the words protein and infection...
proteins and the glutamine repeat containing proteins that cause proteopathies
Proteopathy
In medicine, proteopathy refers to a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body...
or conformational diseases.
Serpin polymerisation causes disease in two ways. First, the lack of active serpin results in uncontrolled protease activity and tissue destruction; this is seen in the case of antitrypsin deficiency. Second, the polymers themselves clog up the endoplasmic reticulum
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
of cells that synthesize serpins, eventually resulting in cell death and tissue damage. In the case of antitrypsin deficiency, antitrypsin polymers cause the death of liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
cells, sometimes resulting in liver damage and cirrhosis
Cirrhosis
Cirrhosis is a consequence of chronic liver disease characterized by replacement of liver tissue by fibrosis, scar tissue and regenerative nodules , leading to loss of liver function...
. Within the cell, serpin polymers are removed via endoplasmic reticulum associated degradation. However, the mechanism by which serpin polymers cause cell death remains to be fully understood.
Like cleaved serpins, serpin polymers are hyperstable with respect to heating, and each serpin monomer appears to have undergone the stressed to relaxed transition. Furthermore, serpin polymers are unable to inhibit target proteases, suggesting that the RCL is unavailable and inserted into the A-sheet. In the absence of definitive structural data, it was, therefore, postulated that serpins polymerise via a mechanism known as A-sheet polymerisation. In normal function the RCL inserts into the A β-sheet to form a fourth strand (figure 4). In the A-sheet polymerisation model, it was suggested that the RCL of one serpin molecule spontaneously inserted into the A-sheet of another, to form a long-chain polymer (figure 9). In effect, it was, thus, proposed that polymerization occurred as a consequence of the requirement of the serpin scaffold to accept an additional β-strand.
Serpins were one of the first families for which disease-causing mutations were directly analyzed in reference to the available crystal structures. In support of the A-sheet polymerisation model, it was noted that many serpin mutations that cause polymerisation localise to two distinct regions of the molecule (highlighted in figure 4a) termed the shutter and the breach. The shutter and the breach contain highly conserved residues, underlie the path of RCL insertion, and are proposed to be important for conformational change.
Two structures of cleaved serpin polymers have been solved; both of which reveal RCL / A-sheet sheet linkages similar to those predicted by the A sheet polymerisation mechanism. However, in direct contrast to the known properties of physiological serpin polymers, crystals of cleaved serpin A-sheet polymers readily dissociate into monomeric forms.

Together, these data have presented an important challenge to the A-sheet model for serpin polymerisation. On the one hand, the idea that serpin polymer formation essentially takes advantage of the serpin mechanism of conformational change is an attractive one. On the other, the biophyiscal data in particular suggest that it is a folding intermediate (rather than the native form) that polymerises, and it is clear that this intermediate must have different structural properties to the native, folded state.
In 2008, a key serpin crystal structure was determined that strongly suggests that physiological serpin polymers do not form via the A-sheet mechanism and instead form via a more extensive domain swapping
Protein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
event. The first such structure solved was of an antithrombin dimer (figure 10), and revealed that both strands s5A and the RCL can be incorporated into the A-sheet of another serpin molecule. This structure can readily be adapted to form long chain polymers. In 2011, the structure of a domain swapped antitrypsin trimer revealed that in polymers of this serpin the RCL is inserted, and that the C-terminal region of the molecule (comprising strands s1C, s4B and s5B) formed the domain swap (figure 11). In support of the physiological relevance of the latter structure, it was shown that antitrypsin polymers formed via a C-terminal domain swap were recognised by a monocloncal antibody specific for pathogenic antitrypsin polymers.
The new "domain swapped" model for serpin polymerisation begins to reconcile the available biophysical and biochemical data. Together, these data suggest that domain swapping events occur when mutations or environmental factors somehow interfere with the final stages of serpin folding to the native state. These data also reveal that different serpins can apparently polymerise via different types of domain swaps. Finally, while these data shed light on the final polymeric form, it is important to note that the precise toxic species of intermediate and / or polymer that causes cell death in, for example, antitrypsin deficiency, remains to be identified.
Mutations that result in spontaneous formation of latent (or latent-like), inactive conformations

It is also worth highlighting a structure of a disease-linked human antichymotrypsin variant that further demonstrates the extraordinary flexibility of the serpin scaffold. The structure of antichymotrypsin (Leucine 55 to Proline) revealed a novel "δ" conformation that may represent an intermediate between the native and latent state (Figure 12). In the delta conformation, four residues of the RCL are inserted into the top of β-sheet A. The bottom half of the sheet is filled as a result of one of the α-helices (the F-helix) partially switching to a β-strand conformation, completing the β-sheet hydrogen bonding. It is unclear whether other serpins can adopt this conformer, and whether this conformation has a functional role. However, it is speculated that the δ-conformation may be adopted by Thyroxine-binding globulin during thyroxine release.
Other mechanisms of serpin-related disease
In humans, simple deficiency of many serpins (e.g., through a null mutation) may result in disease (see Table 1).It is rare that single amino acid changes in the RCL of a serpin alters the specificity of the inhibitor and allow it to target the wrong protease. For example, the Antitrypsin-Pittsburgh mutation (methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
358 to arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
) allowed the serpin to inhibit thrombin
Thrombin
Thrombin is a "trypsin-like" serine protease protein that in humans is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the first step of the coagulation cascade, which ultimately results in the stemming of blood loss...
, thus causing a bleeding
Bleeding
Bleeding, technically known as hemorrhaging or haemorrhaging is the loss of blood or blood escape from the circulatory system...
disorder.
Serpins are suicide inhibitors, the RCL acting as a "bait." Certain disease-linked mutations in the RCL of human serpins permit true substrate-like behaviour and cleavage without complex formation. Such variants are speculated to affect the rate or the extent of RCL insertion into the A-sheet. These mutations, in effect, result in serpin deficiency through a failure to properly control the target protease.
Several non-inhibitory serpins play key roles in important human diseases. For example, maspin functions as a tumour suppressor in breast and prostate cancer. The mechanism of maspin function remains to be fully understood. Murine knockouts
Knockout mouse
A knockout mouse is a genetically engineered mouse in which researchers have inactivated, or "knocked out," an existing gene by replacing it or disrupting it with an artificial piece of DNA...
of maspin are lethal; these data suggest that maspin plays a key role in development.
Evolution
Serpins were initially believed to be restricted to eukaryoteEukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
organisms, but have since been found in a number of bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
. It remains unclear whether these prokaryote genes are the descendants of an ancestral prokaryotic serpin or the product of lateral gene transfer (genetic transfer between organisms not by evolutionary descent). Rawlings et al. showed that serpins are the most widely distributed and largest family of protease inhibitors.
Human
In 2001, a serpin nomenclature was established (see table 1, below). The naming system is based upon a phylogenetic analysis of ~500 serpins.The human genome encodes 16 serpin clades, termed serpinA through to serpinP, encoding 29 inhibitory and 7 non-inhibitory serpin proteins (see Law et al. (2006) for a recent review). The proteins are named serpinXY where X is the clade of the protein and Y the number of the protein within that clade. Table 1 lists each human serpin, together with brief notes in regards to each molecules function and the consequence (where known) of dysfunction or deficiency.
Table 1
| Protein name | PDB | Common Name | Description | Disease / Effect of deficiency | Chromosomal location Locus (genetics) In the fields of genetics and genetic computation, a locus is the specific location of a gene or DNA sequence on a chromosome. A variant of the DNA sequence at a given locus is called an allele. The ordered list of loci known for a particular genome is called a genetic map... |
| SERPINA1 | 1QLP 7API 1D5S |
Alpha 1-antitrypsin Alpha 1-antitrypsin Alpha 1-Antitrypsin or α1-antitrypsin is a protease inhibitor belonging to the serpin superfamily. It is generally known as serum trypsin inhibitor. Alpha 1-antitrypsin is also referred to as alpha-1 proteinase inhibitor because it inhibits a wide variety of proteases... |
extracellular, inhibits human neutrophil elastase. | Deficiency results in emphysema, antitrypsin polymerisation results in cirrhosis. Serpinopathy. The C-terminal fragment of cleaved SERPINA1 may inhibit HIV-1 infection. | 14q32.1 |
| SERPINA2 | Antitrypsin-related protein | extracellular, possible pseudogene | Unknown | 14q32.1 | |
| SERPINA3 | 1YXA 2ACH |
Alpha 1-antichymotrypsin Alpha 1-antichymotrypsin Alpha 1-antichymotrypsin is an alpha globulin glycoprotein that is a member of the serpin superfamily.It inhibits the activity of certain enzymes called proteases, such as cathepsin G that is found in neutrophils, and chymases found in mast cells, by cleaving them into a different shape or... |
Extracellular, inhibits cathepsin G. | Deficiency results in emphysema. Serpinopathy | 14q32.1 |
| SERPINA4 SERPINA4 Kallistatin is a protein that in humans is encoded by the SERPINA4 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
Kallistatin | extracellular, inhibition of kallikrein, regulation of vascular function | Unknown | 14q32.1 | |
| SERPINA5 | 2OL2 3B9F |
Protein C inhibitor Protein C inhibitor Protein C inhibitor is a serine protease inhibitor which limits the expression of protein C .An N-terminal fragment of PCI is a possible serum biomarker for prostate cancer.-Interactions:... |
Extracellular, inhibitor of active protein C Protein C Protein C, also known as autoprothrombin IIA and blood coagulation factor XIV, is a zymogenic protein, the activated form of which plays an important role in regulating blood clotting, inflammation, cell death and maintaining the permeability of blood vessel walls in humans and other animals... . Intracellular role in preventing phagocytosis of bacteria. |
Male murine knockouts are infertile In multiple sclerosis Multiple sclerosis Multiple sclerosis is an inflammatory disease in which the fatty myelin sheaths around the axons of the brain and spinal cord are damaged, leading to demyelination and scarring as well as a broad spectrum of signs and symptoms... , accumulation of PCI has been noted in chronic active plaques. |
14q32.1 |
| SERPINA6 SERPINA6 Corticosteroid-binding globulin also known as transcortin or serpin A6 is a protein that in humans is encoded by the SERPINA6 gene.- Function :... |
2V6D 2VDX 2VDY |
Cortisol binding globulin | Extracellular, non-inhibitory; cortisol binding. | Deficiency may cause chronic fatigue | 14q32.1 |
| SERPINA7 | 2CEO 2RIV 2RIW |
Thyroxine-binding globulin Thyroxine-binding globulin Thyroxine-binding globulin binds thyroid hormone in circulation. It is one of three proteins responsible for carrying the thyroid hormones thyroxine and 3,5,3’-triiodothyronine in the bloodstream. Of these three proteins, TBG has the highest affinity for T4 and T3, but is present in the lowest... |
extracellular, non-inhibitory; thyroxine binding | Deficiency causes hypothyroidism. | Xq22.2 |
| SERPINA8 | 2X0B 2WXW 2WXX 2WXY 2WXZ 2WY0 2WY1 |
Angiotensinogen | Extracellular; non-inhibitory, cleavage by renin results in release of angiotensin I. | Variants linked to hypertension Murine knockouts result in hypotension. | 1q42-q43 |
| SERPINA9 SERPINA9 Serpin A9 also known as centerin or GCET1 is a protein that in humans is encoded by the SERPINA9 gene located on chromosome 14q32.1. Serpin A9 is a member of the serpin family of serine protease inhibitors.- Function :... |
Centerin | Extracellular; inhibitory, maintenance of naive B cells | Unknown | 14q32.1 | |
| SERPINA10 | 3F1S 3H5C |
Protein Z-related protease inhibitor Protein Z-related protease inhibitor Protein Z-dependent protease inhibitor is a protein circulating in the blood which inhibits factors Xa and XIa of the coagulation cascade. It is a member of the class of the serine protease inhibitors... |
extracellular, binds protein Z and inactivates factor Xa and factor XI Factor XI Factor XI or plasma thromboplastin antecedent is the zymogen form of factor XIa, one of the enzymes of the coagulation cascade. Like many other coagulation factors, it is a serine protease. In humans, Factor XI is encoded by the F11 gene.... a) |
Deficiency may cause venous thromboembolic disease | 14q32.1 |
| SERPINA11 | - | probably extracellular, not characterised. | Unknown | 14q32.13 | |
| SERPINA12 | Vaspin | extracellular, insulin-sensitizing adipocytokine | Unknown | 14q32.1 | |
| SERPINA13 | - | probably extracellular, not characterised | Unknown | 14q32 | |
| SERPINB1 SERPINB1 Leukocyte elastase inhibitor also known as serpin B1 is a protein that in humans is encoded by the SERPINB1 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
1HLE | Monocyte neutrophil elastase inhibitor SERPINB1 Leukocyte elastase inhibitor also known as serpin B1 is a protein that in humans is encoded by the SERPINB1 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
Intracellular, inhibition of neutrophil elastase | Murine knockout results in neutrophil survival defect and immune deficiency | 6p25 |
| SERPINB2 | 1BY7 | Plasminogen activator inhibitor-2 Plasminogen activator inhibitor-2 Plasminogen activator inhibitor-2 is a coagulation factor that inactivates tPA and urokinase. It is present in most cells, especially monocytes/macrophages... |
Intracellular/extracellular. Inhibition of extracellular uPA. Intracellular function unclear, however, may protect against viral infection. | Murine knockouts viable / no obvious phenotype | 18q21.3 |
| SERPINB3 SERPINB3 Serpin B3 is a protein that in humans is encoded by the SERPINB3 gene.-Further reading:Turato C, Ruvoletto MG, Biasiolo A, et al. Squamous cell carcinoma antigen-1 polymorphism in chronic liver disease.... |
2ZV6 | Squamous cell carcinoma antigen-1 (SCCA-1) | Intracellular, inhibitor of papain-like cysteine proteases | Unknown | 18q21.3 |
| SERPINB4 SERPINB4 Serpin B4 is a protein that in humans is encoded by the SERPINB4 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
Squamous cell carcinoma antigen-2 (SCCA-2) | Intracellular, inhibitor of cathepsin G and chymase | Unknown | 18q21.3 | |
| SERPINB5 | 1WZ9 | Maspin Maspin Maspin is a serpin and tumor suppressor gene.It has been associated with prostate cancer and breast cancer.It has also been associated with tumors of the pancreas.It was identified in 1994.-Further reading:-External links:... |
Obligate intracellular serpin, non inhibitory, tumour suppressor in breast and prostate cancer | Murine knockouts lethal, important role in cancer metastasis | 18q21.3 |
| SERPINB6 SERPINB6 Serpin B6 is a protein that in humans is encoded by the SERPINB6 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
PI-6 SERPINB6 Serpin B6 is a protein that in humans is encoded by the SERPINB6 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
intracellular, inhibition of cathepsin G | Murine knockout reveals mild neutropenia. In humans, a nonsense mutation in SERPINB6 results in moderate to severe hearing loss. | 6p25 | |
| SERPINB7 SERPINB7 Serpin B7 is a protein that in humans is encoded by the SERPINB7 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
Megsin | intracellular, involved in megakaryocyte maturation | Studies on transgenic mice reveal megsin over-expression causes kidney disease. Histological abnormalities are, however, not apparent in Megsin knockout mice. | 18q21.3 | |
| SERPINB8 SERPINB8 Serpin B8 is a protein that in humans is encoded by the SERPINB8 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
PI-8 SERPINB8 Serpin B8 is a protein that in humans is encoded by the SERPINB8 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
intracellular; possible furin inhibitor | Unknown | 18q21.3 | |
| SERPINB9 SERPINB9 Serpin B9 is a protein that in humans is encoded by the SERPINB9 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
PI-9 SERPINB9 Serpin B9 is a protein that in humans is encoded by the SERPINB9 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
intracellular, inhibitor of the cytotoxic granule protease granzyme B | murine knockout reveals immune dysfunction | 6p25 | |
| SERPINB10 | Bomapin | intracellular, unknown function | Analysis of murine genomic material (C57/BL6; the common lab strain) reveals a stop codon in this gene (BC069938). In contrast, EST data suggests that full length bomapin is expressed in Czech II mice. These data suggest that loss of Bomapin function in mice does not result in an overt phenotype. | 18q21.3 | |
| SERPINB11 | intracellular, unknown function | Murine Serpinb11 is an active inhibitor whereas the human orthalogue is inactive. | 18q21.3 | ||
| SERPINB12 | Yukopin | intracellular, unknown function | Unknown | 18q21.3 | |
| SERPINB13 SERPINB13 Serpin B13 is a protein that in humans is encoded by the SERPINB13 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
Hurpin SERPINB13 Serpin B13 is a protein that in humans is encoded by the SERPINB13 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... /Headpin SERPINB13 Serpin B13 is a protein that in humans is encoded by the SERPINB13 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
intracellular, inhibitor of papain-like cysteine proteases | Unknown | 18q21.3 | |
| SERPINC1 | 2ANT 2ZNH 1AZX 1TB6 2GD4 1T1F |
Antithrombin Antithrombin Antithrombin is a small protein molecule that inactivates several enzymes of the coagulation system. Antithrombin is a glycoprotein produced by the liver and consists of 432 amino acids. It contains three disulfide bonds and a total of four possible glycosylation sites... |
Extracellular, inhibitor of coagulation Coagulation Coagulation is a complex process by which blood forms clots. It is an important part of hemostasis, the cessation of blood loss from a damaged vessel, wherein a damaged blood vessel wall is covered by a platelet and fibrin-containing clot to stop bleeding and begin repair of the damaged vessel... , specifically factor X Factor X Factor X, also known by the eponym Stuart-Prower factor or as prothrombinase, is an enzyme of the coagulation cascade. It is a serine endopeptidase .-Physiology:... , factor IX Factor IX Factor IX is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes hemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to... and thrombin Thrombin Thrombin is a "trypsin-like" serine protease protein that in humans is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the first step of the coagulation cascade, which ultimately results in the stemming of blood loss... |
Deficiency results in thrombosis and other clotting disorders. Serpinopathy | 1q23-q21 |
| SERPIND1 | 1JMJ 1JMO |
Heparin cofactor II Heparin cofactor II Heparin cofactor II, a protein encoded by the SERPIND1 gene, is a coagulation factor that inhibits IIa, and is a cofactor for heparin and dermatan sulfate... |
extracellular, thrombin inhibitor | Murine knockouts are lethal. | 22q11 |
| SERPINE1 | 1DVN 1OC0 |
Plasminogen activator inhibitor 1 | Extracellular; inhibitor of thrombin, uPA and TPa. | Cardiovascular disease, tumour progression | 7q21.3-q22 |
| SERPINE2 SERPINE2 Glia-derived nexin is a protein that in humans is encoded by the SERPINE2 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
Glia derived nexin SERPINE2 Glia-derived nexin is a protein that in humans is encoded by the SERPINE2 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... / Protease nexin I SERPINE2 Glia-derived nexin is a protein that in humans is encoded by the SERPINE2 gene.-External links:* The MEROPS online database for peptidases and their inhibitors:... |
Extracellular, inhibition of uPA and tPA. | Abnormal expression leads to human male infertility. Knockout mice also develop epileptic phenotype. | 2q33-q35 | |
| SERPINF1 SERPINF1 Pigment epithelium-derived factor also known as serpin F1 , is a multifunctional secreted protein that has anti-angiogenic, anti-tumorigenic, and neurotrophic functions. Found in vertebrates, this 50 kDa protein holds promise in the treatment of such conditions as choroidal neovascularization,... |
1IMV | Pigment epithelium derived factor SERPINF1 Pigment epithelium-derived factor also known as serpin F1 , is a multifunctional secreted protein that has anti-angiogenic, anti-tumorigenic, and neurotrophic functions. Found in vertebrates, this 50 kDa protein holds promise in the treatment of such conditions as choroidal neovascularization,... |
Extracellular, non-inhibitory, potent anti-angiogenic molecule. PEDF has been reported to bind the glycosaminoglycan hyaluronan. | Murine knockout studies reveal that SERPINF1 regulates the vasculature and mass of the pancreas and the prostate. Further, SERPINF1 has been demonstrated to promote Notch–dependent renewal of adult periventricular neural stem cells. Human mutations in SERPINF1 cause osteogenesis imperfecta Osteogenesis imperfecta Osteogenesis imperfecta is a genetic bone disorder. People with OI are born with defective connective tissue, or without the ability to make it, usually because of a deficiency of Type-I collagen... type VI. |
17p13.3 |
| SERPINF2 | 2R9Y | Alpha 2-antiplasmin Alpha 2-antiplasmin Alpha 2-antiplasmin is a serine protease inhibitor responsible for inactivating plasmin, an important enzyme that participates in fibrinolysis and degradation of various other proteins... |
extracellular, plasmin inhibitor, inhibitor of fibrinolysis Fibrinolysis Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. This process has two types: primary fibrinolysis and secondary fibrinolysis... . |
Bleeding disorder | 17pter-p12 |
| SERPING1 | 2OAY | Complement 1-inhibitor C1-inhibitor C1-inhibitor is a protease inhibitor belonging to the serpin superfamily. Its main function is the inhibition of the complement system to prevent spontaneous activation. C1-inhibitor is an acute-phase protein that circulates in blood at levels of around 0.25 g/L. The levels rise ~2-fold during... |
Extracellular, C1 esterase inhibitor. | Angiodemia, serpinopathy. Several polymorphisms in the SERPING1 gene are strongly associated with development of age-related macular degeneration and blindness. | 11q11-q13.1 |
| SERPINH1 | 47 kDa Heat shock protein (HSP47) | intracellular, non inhibitory, molecular chaperone in collagen folding. | Murine knockouts are lethal. A missense mutation in human SERPINH1 results in severe osteogenesis imperfecta Osteogenesis imperfecta Osteogenesis imperfecta is a genetic bone disorder. People with OI are born with defective connective tissue, or without the ability to make it, usually because of a deficiency of Type-I collagen... . |
11p15 | |
| SERPINI1 SERPINI1 Neuroserpin is a protein that in humans is encoded by the SERPINI1 gene.It is associated with Familial encephalopathy with neuroserpin inclusion bodies.-Interactions:SERPINI1 has been shown to interact with Tissue plasminogen activator.... |
1JJO 3FGQ 3F5N 3F02 |
Neuroserpin SERPINI1 Neuroserpin is a protein that in humans is encoded by the SERPINI1 gene.It is associated with Familial encephalopathy with neuroserpin inclusion bodies.-Interactions:SERPINI1 has been shown to interact with Tissue plasminogen activator.... |
Extracellular, inhibitor of tPA, uPA and plasmin | Mutated in dementia (FENIB Familial encephalopathy with neuroserpin inclusion bodies Familial encephalopathy with neuroserpin inclusion bodies is a progressive disorder of the nervous system that is characterized by a loss of intellectual functioning and seizures. At first, affected individuals may have difficulty sustaining attention and concentrating. Their judgment, insight,... ). Serpinopathy |
3q26 |
| SERPINI2 SERPINI2 Serpin I2 is a protein that in humans is encoded by the SERPINI2 gene.-External Links:* The MEROPS online database for peptidases and their inhibitors:... |
Pancpin SERPINI2 Serpin I2 is a protein that in humans is encoded by the SERPINI2 gene.-External Links:* The MEROPS online database for peptidases and their inhibitors:... |
Extracellular, unknown protease target. | Studies on the Pequeño mouse line revealed that loss of SERPINI2 results in pancreatic insufficiency through pancreatic acinar cell loss. In addition a possible role for SERPINI2 in inhibition of pancreatic cancer metastasis has been suggested. | 3q26 |
Specialised Mammalian Serpins
Many mammalian serpins have been identified that share no obvious orthology with a human serpin counterpart. Examples include numerous rodent serpins (particularly some of the murine intracellular serpins) as well as the uterine serpins (discussed below).Uterine
The term uterine serpinUterine serpin
Uterine serpins are members of the A clade of the serine proteinase inhibitor superfamily of proteins and are encoded by the SERPINA14 gene. Uterine serpins are produced by the uterine endometrium of a restricted group of mammals under the influence of progesterone or estrogen...
refers to members of the serpin A clade that are encoded by the SERPINA14 gene. Uterine serpins are produced by the uterine endometrium of a restricted group of mammals in the Laurasiatheria clade under the influence of progesterone or estrogen. They are probably not functional proteinase inhibitors and may function during pregnancy to inhibit maternal immune responses against the conceptus or to participate in transplacental transport.
Insect
The Drosophila melanogasterDrosophila melanogaster
Drosophila melanogaster is a species of Diptera, or the order of flies, in the family Drosophilidae. The species is known generally as the common fruit fly or vinegar fly. Starting from Charles W...
genome contains 29 serpin encoding genes. Amino acid sequence analysis has placed 14 of these serpins in serpin clade Q and 3 in serpin clade K with the remaining 12 serpins classified as orphan serpins not belonging to any clade. The clade classification system is difficult to use for Drosophila serpins and instead a nomenclature system has been adopted that is based on the position of Drosphila serpin genes on the Drosophila chromosome
Chromosome
A chromosome is an organized structure of DNA and protein found in cells. It is a single piece of coiled DNA containing many genes, regulatory elements and other nucleotide sequences. Chromosomes also contain DNA-bound proteins, which serve to package the DNA and control its functions.Chromosomes...
s. 13 of the Drosophila serpins occur as isolated genes in the genome (including Serpin-27A, see below), with the remaining 16 organised into three gene clusters that occur at chromosome positions 28D (2 serpins), 42D (5 serpins), 43A (4 serpins), 77B (3 serpins) and 88E (2 serpins).
Studies on Drosophila serpins reveal that Serpin-27A inhibits the Easter protease (the final protease in the Nudel, Gastrulation Defective, Snake and Easter proteolytic cascade) and thus controls dorsoventral patterning. Easter functions to cleave Spätzle (a chemokine-type ligand), which results in Toll
Toll (gene)
The Toll genes encode members of the Toll-like receptor class of proteins. "Toll" is German for "amazing" or "great". Mutants in the Toll gene were originally identified by 1995 Nobel Laureates Christiane Nüsslein-Volhard and Eric Wieschaus and colleagues in the fruit fly Drosophila melanogaster...
mediated signaling. In addition to its central role in embryonic patterning, Toll signaling is also important for the innate immune response in insects. Accordingly, serpin-27A additionally functions to control the insect immune response. Interestingly, in Tenebrio molitor (a large beetle), a protein (SPN93) comprising two discrete tandem serpin domains functions to regulate the toll proteolytic cascade.
Worm
The genome of the nematode worm C. elegansCaenorhabditis elegans
Caenorhabditis elegans is a free-living, transparent nematode , about 1 mm in length, which lives in temperate soil environments. Research into the molecular and developmental biology of C. elegans was begun in 1974 by Sydney Brenner and it has since been used extensively as a model...
contains nine serpins, however, only five of these molecules appear to function as protease inhibitors. One of these serpins, SRP-6, has been shown to perform a protective function and guard against stress induced calpain-associated lysosomal disruption. Further SRP-6 functions to inhibit lysosomal cysteine proteases released after lysosomal rupture. Accordingly, worms lacking SRP-6 are sensitive to stress. Most notably, SRP-6 knockout worms die when placed in water (the hypo-osmotic stress lethal phenotype or Osl). Based on these data it is suggested that lysosomes play a general and controllable role in determining cell fate.
Plant
The presence of serpins in plants has long been recognised, indeed, an abundant barley grain serpin (barley Protein Z) is one of the major protein components in beer.The MEROPS database identifies 18 serpin family members in the Arabidopsis thaliana
Arabidopsis thaliana
Arabidopsis thaliana is a small flowering plant native to Europe, Asia, and northwestern Africa. A spring annual with a relatively short life cycle, arabidopsis is popular as a model organism in plant biology and genetics...
genome, but only about eight of these are full-length serpin sequences. Plant serpins are potent inhibitors of mammalian chymotrypsin-like serine proteases in vitro, the most well-studied example being barley serpin Zx (BSZx), which is able to inhibit trypsin, chymotrypsin as well as several blood coagulation factors. However, close relatives of chymotrypsin-like serine proteases are absent in plants. Interestingly, the RCL of several serpins from wheat grain and rye contain poly-Q repeat sequences similar to those present in the prolamin storage proteins of the endosperm. It has therefore been suggested that plant serpins may function to inhibit proteases from insects or microbes that cleave grain storage proteins. In support of this hypothesis, specific plant serpins have been identified in the phloem sap of pumpkin (CmPS-1) and cucumber plants. However, while an inverse correlation between up-regulation of CmPS-1 expression and aphid survival was observed, in vitro feeding experiments revealed that recombinant CmPS-1 did not appear to affect insect survival.
Alternative roles and protease targets for plant serpins have been proposed. It has been shown that Arabidopsis AtSerpin1 (At1g47710; 3LE2) inhibits metacaspase
Metacaspase
Metacaspases are related to caspases and paracaspase. The metacaspases are Arginine/Lysine-specific, in contrast to caspases, which are Aspartate-specific...
-like proteases in vitro. More recently the major in vivo protease target for AtSerpin1 was identified as the papain-like cysteine protease RESPONSIVE TO DESICCATION-21 (RD21). In the same study the X-ray crystal structure of the native, stressed form of AtSerpin1 was shown to have plant-specific features. Two other Arabidopsis serpins, AtSRP2 (At2g14540) and AtSRP3 (At1g64030) appear to be involved in responses to DNA damage caused by plant exposure to methane methylsulfonate (MMS).
Fungal
A single fungal serpin has been characterized to date: celpin from PiromycesNeocallimastigomycota
Neocallimastigomycota is a phylum of anaerobic fungi, found in the digestive tracts of herbivores. It encompasses only one family.-Discovery:...
sp. strain E2. Piromyces is an anaerobic fungus found in the gut of ruminants and is important for digesting plant material. Celpin is predicted to be an inhibitory molecule and contains two N-terminal dockerin domains in addition to the serpin domain. Dockerins are commonly found in proteins that localise to the fungal cellulosome, a large extracellular mulitprotein complex that breaks down cellulose. It is therefore suggested that celpin protects the cellulosome against plant proteases. Interestingly certain bacterial serpins also localize to the cellulosome.
Prokaryote
Predicted serpin genes are sporadically distributed in prokaryotes. In vitro studies on some of these moelcules have revealed that they are able to inhibit proteases and it is suggested that they function as inhibitors in vivo. Interestingly, several prokaryote serpins are found in extremophiles. Accordingly, and in contrast to mammalian serpins, these molecule possess elevated resistance to heat denaturation. The precise role of most bacterial serpins remains obscure, however, ClostridiumClostridium
Clostridium is a genus of Gram-positive bacteria, belonging to the Firmicutes. They are obligate anaerobes capable of producing endospores. Individual cells are rod-shaped, which gives them their name, from the Greek kloster or spindle...
thermocellum serpin localises to the cellulosome. It is suggested that the role of cellulosome-associated serpins may be to prevent unwanted protease activity against the cellulosome.
Viral
Serpins are also expressed by viruses as a way to evade the host's immune defense. In particular, serpins expressed by pox viruses, including cow pox (vaccinia) and rabbit pox (myxoma), are of interest because of their potential use as novel therapeutics for immune and inflammatory disorders as well as transplant therapy. A study on Serp1 reveals this molecule suppresses the Toll-mediated innate immune response and allows indefinite cardiac allograft survival in rats. Studies on Crma and Serp2, reveal both are cross-class inhibitor and targets both serine (Granzyme B; albeit weakly) and cysteine proteases (Caspase 1 and Caspase 8). In comparison to their mammalian counterparts, viral serpins contain significant deletions of elements of secondary structure. Specifically, structural studies on crmA reveals this molecule lacks the D-helix as well as significant portions of the A- and E-helices.See also
- Wikipedia:MeSH D12.776#MeSH D12.776.872 --- serpins
- ProteopathyProteopathyIn medicine, proteopathy refers to a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body...
- Familial encephalopathy with neuroserpin inclusion bodiesFamilial encephalopathy with neuroserpin inclusion bodiesFamilial encephalopathy with neuroserpin inclusion bodies is a progressive disorder of the nervous system that is characterized by a loss of intellectual functioning and seizures. At first, affected individuals may have difficulty sustaining attention and concentrating. Their judgment, insight,...
External links
- James Whisstock laboratory at Monash UniversityMonash UniversityMonash University is a public university based in Melbourne, Victoria. It was founded in 1958 and is the second oldest university in the state. Monash is a member of Australia's Group of Eight and the ASAIHL....
- Jim Huntington serpin laboratory at University of CambridgeUniversity of CambridgeThe University of Cambridge is a public research university located in Cambridge, United Kingdom. It is the second-oldest university in both the United Kingdom and the English-speaking world , and the seventh-oldest globally...
- Frank Church serpin group at University of North Carolina at Chapel HillUniversity of North Carolina at Chapel HillThe University of North Carolina at Chapel Hill is a public research university located in Chapel Hill, North Carolina, United States...
- Paul Declerck serpin group at Katholieke Universiteit LeuvenKatholieke Universiteit LeuvenThe Katholieke Universiteit Leuven is a Dutch-speaking university in Flanders, Belgium.It is located at the centre of the historic town of Leuven, and is a prominent part of the city, home to the university since 1425...
- Tom Roberts laboratory at Macquarie UniversityMacquarie UniversityMacquarie University is an Australian public teaching and research university located in Sydney, with its main campus situated in Macquarie Park. Founded in 1964 by the New South Wales Government, it was the third university to be established in the metropolitan area of Sydney...
- Robert Fluhr laboratory at Weizmann Institute of ScienceWeizmann Institute of ScienceThe Weizmann Institute of Science , known as Machon Weizmann, is a university and research institute in Rehovot, Israel. It differs from other Israeli universities in that it offers only graduate and post-graduate studies in the sciences....
- Merops general protease / inhibitor classification page at University of CambridgeUniversity of CambridgeThe University of Cambridge is a public research university located in Cambridge, United Kingdom. It is the second-oldest university in both the United Kingdom and the English-speaking world , and the seventh-oldest globally...
- The alpha one foundation provides information and support for sufferers of antitrypsin deficiency

