S-process
Encyclopedia
The S-process or slow-neutron-capture-process is a nucleosynthesis
process that occurs at relatively low neutron density and intermediate temperature conditions in star
s. Under these conditions the rate of neutron capture
by atomic nuclei
is slow relative to the rate of radioactive beta-minus decay
. In the S-process, a stable isotope captures a neutron, but the radioactive isotope that results decays to its stable daughter before the next neutron is captured. This process produces stable isotopes by moving along the valley of beta-decay stable isobars
in the chart of isotopes
. The S-process produces approximately half of the isotopes of the elements heavier than iron
, and therefore plays an important role in the galactic chemical evolution
. The S-process differs from the more rapid R-process
of neutron capture by its slow rate of neutron captures.
by Hans Suess
and Harold Urey
in 1956. Among other things, this data showed abundance peaks for strontium
, barium
, and lead
, which, according to quantum mechanics
and the nuclear shell model, are particularly stable nuclei, much like the noble gas
es are chemically inert
. This implied that some abundant nuclei must be created by slow neutron capture
, and it was only a matter of determining what other nuclei could be accounted for by such a process. A table apportioning the heavy isotopes between S-process and R-process
was published in the famous B2FH review paper in 1957. There it was also argued that the S-process occurs in red giant
stars. In a particularly illustrative case, the element technetium
, with a longest half-life of 4.2 million years, had been discovered in S-, M-, and N-type stars in 1952. Since these stars were thought to be billions of years old, the presence of technetium in their outer atmospheres was taken as evidence of its recent creation there, unconnected with events in the deep interior of the star in the region of active fusion, or events in the star's early history billions of years in the past.
A calculable model for creating the heavy isotopes from iron seed nuclei in a time-dependent manner was not provided until 1961. That work showed that the large overabundances of barium observed by astronomers in certain red-giant stars could be created from iron seed nuclei if the total fluence (number of neutrons per unit area) of neutrons was appropriate. It also showed that no one single fluence could account for the observed S-process abundances, but that a wide range of fluences is required. The numbers of iron seed nuclei that were exposed to a given fluence must decrease as the fluence becomes stronger. This work also showed that the curve of the product of neutron-capture cross section times abundance is not a smoothly falling curve, but rather has a ledge-precipice structure. A series of papers in the 1970s by D. Clayton based on the assumption of an exponentially declining neutron fluence as a function of the number of iron seed so exposed became the standard model of the S-process and remained so until the details of AGB-star
nucleosynthesis became advanced enough that they became a standard model based on the stellar structure models. Important series of measurements of neutron-capture cross sections were reported from Oak Ridge National Lab in 1965
and by Karlsruhe Nuclear Physics Center in 1982
and subsequently. These placed the S-process on the firm quantitative basis that it enjoys today.
stars. In contrast to the R-process which is believed to occur over time scales of seconds in explosive environments, the S-process is believed to occur over time scales of thousands of years. The extent to which the s-process moves up the elements in the chart of isotopes to higher mass number
s is essentially determined by the degree to which the star in question is able to produce neutron
s, and by the amount of iron in the star's initial abundance distribution. Iron
is the "starting material" (or seed) for this neutron capture - beta-minus decay sequence of synthesizing new elements.
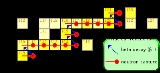
The main neutron source
reactions are:
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|}
 One distinguishes the main and the weak s-process component. The main component produces heavy elements beyond Sr
One distinguishes the main and the weak s-process component. The main component produces heavy elements beyond Sr
and Y
, and up to Pb
in the lowest metallicity stars. The production site of the main component are low-mass Asymptotic Giant Branch
stars. The weak component of the S-process, on the other hand, synthesizes S-process isotopes of elements from the iron group up to Sr and Y, and takes place at the end of He
- and C
-burning in massive stars. These stars will become supernovae at their demise and spew those s isotopes into interstellar space.
The S-process is often mathematically treated using the so-called local approximation, which gives a theoretical model of elemental abundances based on the assumption of constant neutron flux in a star, so that the ratio of abundances is inversely proportional to the ratio of neutron-capture cross-sections for different isotopes. This approximation is - as the name indicates - only valid locally, meaning for isotopes of similar mass number.
Because of the relatively low neutron flux
es expected to occur during the S-process (on the order of 105 to 1011 neutrons per cm2 per second), this process does not have the ability to produce any of the heavy radioactive isotopes such as thorium
or uranium
. The cycle that terminates the S-process is:
captures a neutron, producing , which decays to by β- decay
. in turn decays to by α decay
:
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|- style="height:2em;"
| || || ||→ || ||+ || ||+ ||
|- style="height:2em;"
| || || ||→ || ||+ ||
|}
then captures three neutrons, producing , which decays to by β- decay, restarting the cycle:
|- style="height:2em;"
| ||+ ||3 ||→ ||
|- style="height:2em;"
| || || ||→ || ||+ || ||+ ||
|}
The net result of this cycle therefore is that 4 neutron
s are converted into one alpha particle
, two electron
s, two anti-electron neutrino
s and gamma radiation
:
|- style="height:2em;"
| || ||4 ||→ || ||+ ||2 ||+ ||2 ||+ ||
|}
The process thus terminates in bismuth, the heaviest "stable" element. (Bismuth is actually slightly radioactive, but with a half-life so long—a billion times the present age of the universe—that it is effectively stable over the lifetime of any existing star.)
. Individual solid grains from various long-dead stars that existed before the solar system are found in meteorites, where they have been preserved. The origin of these grains is demonstrated by laboratory measurements of extremely unusual isotopic abundance ratios within the grain. The results give new insight into astrophysics. Silicon-carbide (SiC) grains condense in the atmospheres of AGB stars and thus trap the isotopes of that star. Because the AGB stars are the main site of the S-process in the galaxy, the heavy elements in the SiC grains are virtually pure S-process isotopes. This fact has been demonstrated repeatedly by sputtering-ion mass spectrometer studies of these presolar grains
. Several surprising results have shown that the ratio of S-process and R-process abundances is somewhat different from that which was previously assumed. It has also been shown with trapped isotopes of krypton and xenon that the S-process abundances in the stellar atmospheres change with time or from star to star, presumably with the strength of neutron fluence or perhaps the temperature. This is a frontier of S-process studies today.
Nucleosynthesis
Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons . It is thought that the primordial nucleons themselves were formed from the quark–gluon plasma from the Big Bang as it cooled below two trillion degrees...
process that occurs at relatively low neutron density and intermediate temperature conditions in star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s. Under these conditions the rate of neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
by atomic nuclei
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
is slow relative to the rate of radioactive beta-minus decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
. In the S-process, a stable isotope captures a neutron, but the radioactive isotope that results decays to its stable daughter before the next neutron is captured. This process produces stable isotopes by moving along the valley of beta-decay stable isobars
Beta-decay stable isobars
Beta-decay stable isobars are the set of nuclides which cannot undergo beta decay, that is, the transformation of a neutron to a proton or a proton to a neutron within the nucleus...
in the chart of isotopes
Table of nuclides
The tables listed below provide information on the basic properties of all nuclides.* Neutron + Element 1 - Element 24 * Element 25 - Element 48 * Element 49 - Element 72...
. The S-process produces approximately half of the isotopes of the elements heavier than iron
Heavy metals
A heavy metal is a member of a loosely-defined subset of elements that exhibit metallic properties. It mainly includes the transition metals, some metalloids, lanthanides, and actinides. Many different definitions have been proposed—some based on density, some on atomic number or atomic weight,...
, and therefore plays an important role in the galactic chemical evolution
Nucleosynthesis
Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons . It is thought that the primordial nucleons themselves were formed from the quark–gluon plasma from the Big Bang as it cooled below two trillion degrees...
. The S-process differs from the more rapid R-process
R-process
The r-process is a nucleosynthesis process, likely occurring in core-collapse supernovae responsible for the creation of approximately half of the neutron-rich atomic nuclei that are heavier than iron. The process entails a succession of rapid neutron captures on seed nuclei, typically Ni-56,...
of neutron capture by its slow rate of neutron captures.
History
The S-process was seen to be needed from the relative abundances of isotopes of heavy elements and from a newly published table of abundancesAbundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
by Hans Suess
Hans Suess
Hans Eduard Suess was an Austrian physical chemist and nuclear physicist. He was a grandson of the Austrian geologist Eduard SuessSuess earned his Ph.D. in chemistry from the University of Vienna in 1935...
and Harold Urey
Harold Urey
Harold Clayton Urey was an American physical chemist whose pioneering work on isotopes earned him the Nobel Prize in Chemistry in 1934...
in 1956. Among other things, this data showed abundance peaks for strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
, barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
, and lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, which, according to quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
and the nuclear shell model, are particularly stable nuclei, much like the noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es are chemically inert
Inert
-Chemistry:In chemistry, the term inert is used to describe a substance that is not chemically reactive.The noble gases were previously known as inert gases because of their perceived lack of participation in any chemical reactions...
. This implied that some abundant nuclei must be created by slow neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
, and it was only a matter of determining what other nuclei could be accounted for by such a process. A table apportioning the heavy isotopes between S-process and R-process
R-process
The r-process is a nucleosynthesis process, likely occurring in core-collapse supernovae responsible for the creation of approximately half of the neutron-rich atomic nuclei that are heavier than iron. The process entails a succession of rapid neutron captures on seed nuclei, typically Ni-56,...
was published in the famous B2FH review paper in 1957. There it was also argued that the S-process occurs in red giant
Red giant
A red giant is a luminous giant star of low or intermediate mass in a late phase of stellar evolution. The outer atmosphere is inflated and tenuous, making the radius immense and the surface temperature low, somewhere from 5,000 K and lower...
stars. In a particularly illustrative case, the element technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
, with a longest half-life of 4.2 million years, had been discovered in S-, M-, and N-type stars in 1952. Since these stars were thought to be billions of years old, the presence of technetium in their outer atmospheres was taken as evidence of its recent creation there, unconnected with events in the deep interior of the star in the region of active fusion, or events in the star's early history billions of years in the past.
A calculable model for creating the heavy isotopes from iron seed nuclei in a time-dependent manner was not provided until 1961. That work showed that the large overabundances of barium observed by astronomers in certain red-giant stars could be created from iron seed nuclei if the total fluence (number of neutrons per unit area) of neutrons was appropriate. It also showed that no one single fluence could account for the observed S-process abundances, but that a wide range of fluences is required. The numbers of iron seed nuclei that were exposed to a given fluence must decrease as the fluence becomes stronger. This work also showed that the curve of the product of neutron-capture cross section times abundance is not a smoothly falling curve, but rather has a ledge-precipice structure. A series of papers in the 1970s by D. Clayton based on the assumption of an exponentially declining neutron fluence as a function of the number of iron seed so exposed became the standard model of the S-process and remained so until the details of AGB-star
Asymptotic Giant Branch
The asymptotic giant branch is the region of the Hertzsprung-Russell diagram populated by evolving low to medium-mass stars. This is a period of stellar evolution undertaken by all low to intermediate mass stars late in their lives....
nucleosynthesis became advanced enough that they became a standard model based on the stellar structure models. Important series of measurements of neutron-capture cross sections were reported from Oak Ridge National Lab in 1965
and by Karlsruhe Nuclear Physics Center in 1982
and subsequently. These placed the S-process on the firm quantitative basis that it enjoys today.
The S-process in stars
The S-process is believed to occur mostly in Asymptotic Giant BranchAsymptotic Giant Branch
The asymptotic giant branch is the region of the Hertzsprung-Russell diagram populated by evolving low to medium-mass stars. This is a period of stellar evolution undertaken by all low to intermediate mass stars late in their lives....
stars. In contrast to the R-process which is believed to occur over time scales of seconds in explosive environments, the S-process is believed to occur over time scales of thousands of years. The extent to which the s-process moves up the elements in the chart of isotopes to higher mass number
Mass number
The mass number , also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion...
s is essentially determined by the degree to which the star in question is able to produce neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s, and by the amount of iron in the star's initial abundance distribution. Iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
is the "starting material" (or seed) for this neutron capture - beta-minus decay sequence of synthesizing new elements.
The main neutron source
Neutron source
A Neutron source is a device that emits neutrons. There is a wide variety of different sources, ranging from hand-held radioactive sources to neutron research facilities operating research reactors and spallation sources...
reactions are:
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|}

Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
and Y
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
, and up to Pb
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
in the lowest metallicity stars. The production site of the main component are low-mass Asymptotic Giant Branch
Asymptotic Giant Branch
The asymptotic giant branch is the region of the Hertzsprung-Russell diagram populated by evolving low to medium-mass stars. This is a period of stellar evolution undertaken by all low to intermediate mass stars late in their lives....
stars. The weak component of the S-process, on the other hand, synthesizes S-process isotopes of elements from the iron group up to Sr and Y, and takes place at the end of He
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
- and C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-burning in massive stars. These stars will become supernovae at their demise and spew those s isotopes into interstellar space.
The S-process is often mathematically treated using the so-called local approximation, which gives a theoretical model of elemental abundances based on the assumption of constant neutron flux in a star, so that the ratio of abundances is inversely proportional to the ratio of neutron-capture cross-sections for different isotopes. This approximation is - as the name indicates - only valid locally, meaning for isotopes of similar mass number.
Because of the relatively low neutron flux
Neutron flux
The neutron flux is a quantity used in reactor physics corresponding to the total length travelled by all neutrons per unit time and volume . The neutron fluence is defined as the neutron flux integrated over a certain time period....
es expected to occur during the S-process (on the order of 105 to 1011 neutrons per cm2 per second), this process does not have the ability to produce any of the heavy radioactive isotopes such as thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
or uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
. The cycle that terminates the S-process is:
captures a neutron, producing , which decays to by β- decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
. in turn decays to by α decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
:
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|- style="height:2em;"
| || || ||→ || ||+ || ||+ ||
|- style="height:2em;"
| || || ||→ || ||+ ||
|}
then captures three neutrons, producing , which decays to by β- decay, restarting the cycle:
- {| border="0"
|- style="height:2em;"
| ||+ ||3 ||→ ||
|- style="height:2em;"
| || || ||→ || ||+ || ||+ ||
|}
The net result of this cycle therefore is that 4 neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s are converted into one alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
, two electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s, two anti-electron neutrino
Neutrino
A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected...
s and gamma radiation
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
:
- {| border="0"
|- style="height:2em;"
| || ||4 ||→ || ||+ ||2 ||+ ||2 ||+ ||
|}
The process thus terminates in bismuth, the heaviest "stable" element. (Bismuth is actually slightly radioactive, but with a half-life so long—a billion times the present age of the universe—that it is effectively stable over the lifetime of any existing star.)
The S-process measured in stardust
Stardust is one component of cosmic dustCosmic dust
Cosmic dust is a type of dust composed of particles in space which are a few molecules to 0.1 µm in size. Cosmic dust can be further distinguished by its astronomical location; for example: intergalactic dust, interstellar dust, interplanetary dust and circumplanetary dust .In our own Solar...
. Individual solid grains from various long-dead stars that existed before the solar system are found in meteorites, where they have been preserved. The origin of these grains is demonstrated by laboratory measurements of extremely unusual isotopic abundance ratios within the grain. The results give new insight into astrophysics. Silicon-carbide (SiC) grains condense in the atmospheres of AGB stars and thus trap the isotopes of that star. Because the AGB stars are the main site of the S-process in the galaxy, the heavy elements in the SiC grains are virtually pure S-process isotopes. This fact has been demonstrated repeatedly by sputtering-ion mass spectrometer studies of these presolar grains
Presolar grains
Presolar grains are isotopically-distinct clusters of material found in the fine-grained matrix of primitive meteorites, such as chondrites, whose differences from the surrounding meteorite suggest that they are older than the solar system...
. Several surprising results have shown that the ratio of S-process and R-process abundances is somewhat different from that which was previously assumed. It has also been shown with trapped isotopes of krypton and xenon that the S-process abundances in the stellar atmospheres change with time or from star to star, presumably with the strength of neutron fluence or perhaps the temperature. This is a frontier of S-process studies today.

