Rifamycin
Encyclopedia
The rifamycins are a group of antibiotic
s that are synthesized either naturally by the bacterium Amycolatopsis mediterranei or artificially. They are a subclass of the larger family Ansamycin
. Rifamycins are particularly effective against mycobacteria, and are therefore used to treat tuberculosis
, leprosy
, and mycobacterium avium complex
(MAC) infections.
The rifamycin group includes the "classic" rifamycin drugs as well as the rifamycin derivatives rifampicin
(or rifampin), rifabutin
, rifapentine
and rifalazil.
in southern France. The name was originally given by two microbiologists working with the Italian drug company Group Lepetit SpA in Milan
, the Italian Grazia Beretta, and Pinhas Margalith of Israel.
In 1969, the bacterium was renamed Nocardia mediterranei when another scientist named Thiemann found that it has a cell wall typical of the Nocardia species. Then, in 1986, the bacterium was renamed again Amycolatopsis mediterranei, as the first species of a new genus, because a scientist named Lechevalier discovered that the cell wall lacks mycolic acid
and is not able to be infected by the Nocardia and Rhodococcus phages.
Based on 16S rRNA sequences, Bala et al. renamed the species in 2004 Amycolatopsis rifamycinica.
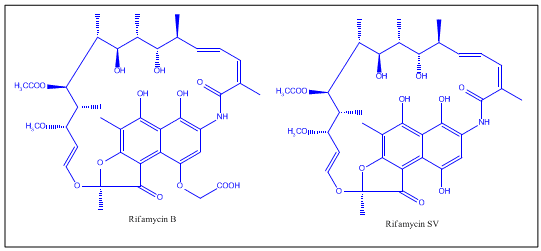
Of the various rifamycins, Rifamycin B was the first introduced commercially. Lepetit filed for patent protection of Rifamycin B in the UK in August 1958, and in the US in March 1959. The British patent GB921045 was granted in March 1963, and U.S. Patent 3,150,046 was granted in September 1964. The drug is widely regarded as having helped conquer the issue of drug-resistant tuberculosis in the 1960s.
. Crystal structure data of the antibiotic bound to RNA polymerase indicates that rifamycin blocks synthesis by causing strong steric clashes with the growing oligonucleotide. If rifamycin binds the polymerase after the chain elongation process has started, no effect is observed on the biosynthesis, which is consistent with a model that suggests rifamycin physically blocks chain elongation. In addition, rifamycins showed potency towards HIV. This is due to their inhibition of the enzyme reverse transcriptase, which is essential for virulence persistence. However, rifamycin's potency proved to be mild, and this never lead to their introduction to clinical trials.
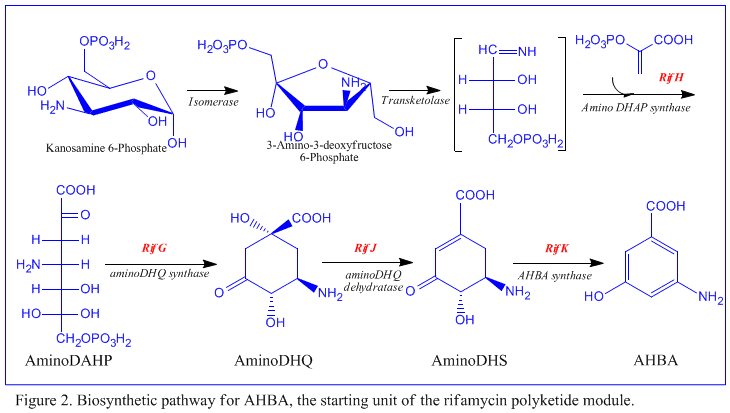
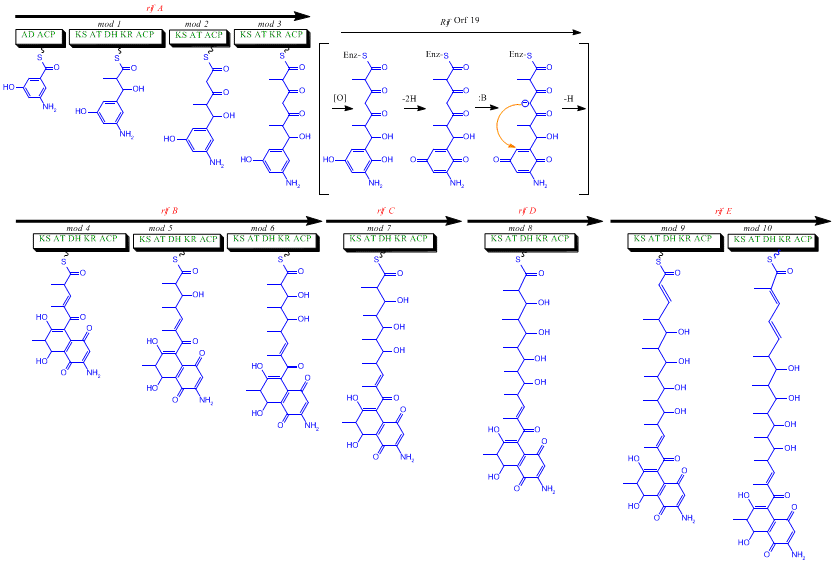
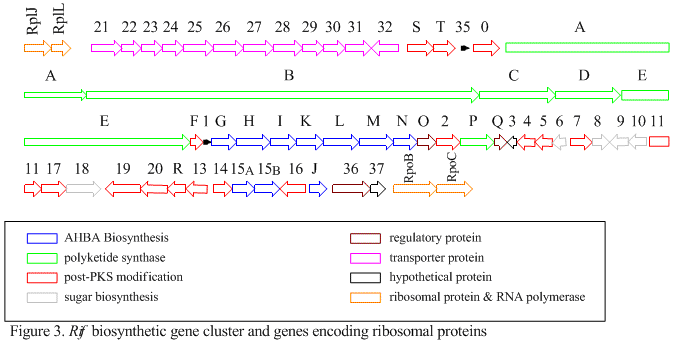
The rif cluster is responsible for the biosynthesis of rifamycins. It contains genes rifG through rifN, which were shown to biosynthesize AHBA.[10] RifK, rifL, rifM, and rifN are believed to act as transaminases in order to form the AHBA precursor kanosamine. "RifH" encodes aminoDAHP synthase that catalyzes the condensation between 1-deoxy-1-imino-d-erythrose 4-phosphate and phosphoenolpyruvate. RifA through rifE encode a type I polyketide synthase module, with the loading module being a non-ribosomal peptide synthase. In all, rifA-E assemble a linear undecaketide and are followed by rifF, which encodes an amide synthase and causes the undecaketide to release and form a macrolactam structure. Moreover, the rif cluster contains various regulatory proteins and glycosylating genes that appear to be silent. Other types of genes seem to perform post-synthase modifications of the original polyketide.
) introduced rifapentine in 1999.
Rifaximin is an oral rifamycin marketed in the US by Salix Pharmaceuticals that is not absorbed from the intestine. It is intended to treat intestinal infections due to Escherichia coli
.
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
s that are synthesized either naturally by the bacterium Amycolatopsis mediterranei or artificially. They are a subclass of the larger family Ansamycin
Ansamycin
Ansamycins is a family of secondary metabolites that show antimicrobial activity against many gram-positive and some gram-negative bacteria and includes various compounds, among which: streptovaricins and rifamycins...
. Rifamycins are particularly effective against mycobacteria, and are therefore used to treat tuberculosis
Tuberculosis
Tuberculosis, MTB, or TB is a common, and in many cases lethal, infectious disease caused by various strains of mycobacteria, usually Mycobacterium tuberculosis. Tuberculosis usually attacks the lungs but can also affect other parts of the body...
, leprosy
Leprosy
Leprosy or Hansen's disease is a chronic disease caused by the bacteria Mycobacterium leprae and Mycobacterium lepromatosis. Named after physician Gerhard Armauer Hansen, leprosy is primarily a granulomatous disease of the peripheral nerves and mucosa of the upper respiratory tract; skin lesions...
, and mycobacterium avium complex
Mycobacterium avium complex
Mycobacterium avium complex is a group of genetically related bacteria belonging to the genus Mycobacterium. It includes Mycobacterium avium and Mycobacterium intracellulare....
(MAC) infections.
The rifamycin group includes the "classic" rifamycin drugs as well as the rifamycin derivatives rifampicin
Rifampicin
Rifampicin or rifampin is a bactericidal antibiotic drug of the rifamycin group. It is a semisynthetic compound derived from Amycolatopsis rifamycinica ...
(or rifampin), rifabutin
Rifabutin
Rifabutin is a bactericidal antibiotic drug primarily used in the treatment of tuberculosis. The drug is a semi-synthetic derivative of rifamycin S. Its effect is based on blocking the DNA-dependent RNA-polymerase of the bacteria. It is effective against Gram-positive and some Gram-negative...
, rifapentine
Rifapentine
Rifapentine is an antibiotic drug used in the treatment of tuberculosis.-History:...
and rifalazil.
Bacterium
Streptomyces mediterranei was first isolated in 1957 from a soil sample collected near the beach-side town of St RaphaelSaint-Raphaël, Var
Saint-Raphaël is a commune in the Var department in the Provence-Alpes-Côte d'Azur region in southeastern France.Immediately to the west of Saint-Raphaël lies another, older, town called Fréjus, and together they form an urban agglomeration known as Fréjus Saint-Raphaël...
in southern France. The name was originally given by two microbiologists working with the Italian drug company Group Lepetit SpA in Milan
Milan
Milan is the second-largest city in Italy and the capital city of the region of Lombardy and of the province of Milan. The city proper has a population of about 1.3 million, while its urban area, roughly coinciding with its administrative province and the bordering Province of Monza and Brianza ,...
, the Italian Grazia Beretta, and Pinhas Margalith of Israel.
In 1969, the bacterium was renamed Nocardia mediterranei when another scientist named Thiemann found that it has a cell wall typical of the Nocardia species. Then, in 1986, the bacterium was renamed again Amycolatopsis mediterranei, as the first species of a new genus, because a scientist named Lechevalier discovered that the cell wall lacks mycolic acid
Mycolic acid
Mycolic acids are long fatty acids found in the cell walls of the mycolata taxon, a group of bacteria that includes Mycobacterium tuberculosis, the causative agent of the disease tuberculosis. They form the major component of the cell wall of mycolata species...
and is not able to be infected by the Nocardia and Rhodococcus phages.
Based on 16S rRNA sequences, Bala et al. renamed the species in 2004 Amycolatopsis rifamycinica.
First drugs
Rifamycins were first isolated in 1957 from a fermentation culture of Streptomyces mediterranei at the laboratory of Gruppo Lepetit SpA in Milan by two scientist named Piero Sensi and Maria Teresa Timbal, working with the Israeli scientist Pinhas Margalith. Eventually, around seven rifamycins were discovered, named Rifamycin A, B, C, D, E, S, and SV.
Of the various rifamycins, Rifamycin B was the first introduced commercially. Lepetit filed for patent protection of Rifamycin B in the UK in August 1958, and in the US in March 1959. The British patent GB921045 was granted in March 1963, and U.S. Patent 3,150,046 was granted in September 1964. The drug is widely regarded as having helped conquer the issue of drug-resistant tuberculosis in the 1960s.
Clinical trials
Rifamycins have been used for the treatment of many diseases, the most important one being HIV-related Tuberculosis. Due to the large number of available analogues and derivatives, rifamycins have been widely utilized in the elimination of pathogenic bacteria that have become resistant to commonly used antibiotics. For instance, Rifampicin is known for its potent effect and ability to prevent drug resistance. It rapidly kills fast-dividing bacilli strains as well as “persisters” cells, which remain biologically inactive for long periods of time that allow them to evade antibiotic activity. In addition, rifabutin and rifapentine have both been used against tuberculosis acquired in HIV-positive patients.Mechanism of action
The biological activity of rifamycins relies on the inhibition of DNA-dependent RNA synthesis. This is due to the high affinity of rifamycins to prokaryotic RNA polymeraseRNA polymerase
RNA polymerase is an enzyme that produces RNA. In cells, RNAP is needed for constructing RNA chains from DNA genes as templates, a process called transcription. RNA polymerase enzymes are essential to life and are found in all organisms and many viruses...
. Crystal structure data of the antibiotic bound to RNA polymerase indicates that rifamycin blocks synthesis by causing strong steric clashes with the growing oligonucleotide. If rifamycin binds the polymerase after the chain elongation process has started, no effect is observed on the biosynthesis, which is consistent with a model that suggests rifamycin physically blocks chain elongation. In addition, rifamycins showed potency towards HIV. This is due to their inhibition of the enzyme reverse transcriptase, which is essential for virulence persistence. However, rifamycin's potency proved to be mild, and this never lead to their introduction to clinical trials.
Biosynthesis
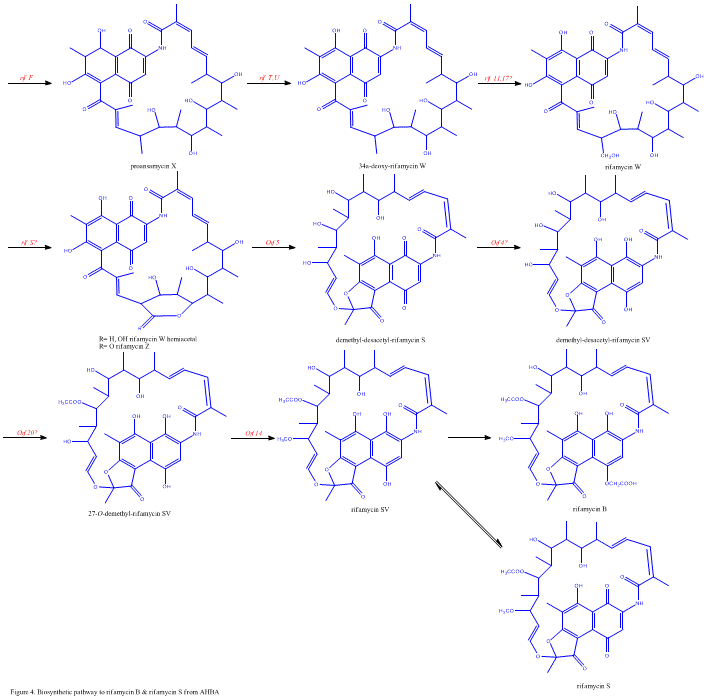
Despite the fact that Rifamycin B is a mild antibacterial compound, it is known to be the precursor of various other clinically-utilized potent derivatives. The general scheme of biosynthesis starts with the uncommon starting unit, 3-amino-5-dihydroxybenzoic acid (AHBA), via type I polyketide pathway (PKS I) in which chain extension is performed using 2 acetate and 8 propionate units. AHBA is believed to have originated from the Shikimate pathway, however this was not incorporated into the biosynthetic mechanism. This is due to the observation that 3 amino-acid analogues converted into AHBA in cell-free extracts of A. mediterranei.


The rif cluster is responsible for the biosynthesis of rifamycins. It contains genes rifG through rifN, which were shown to biosynthesize AHBA.[10] RifK, rifL, rifM, and rifN are believed to act as transaminases in order to form the AHBA precursor kanosamine. "RifH" encodes aminoDAHP synthase that catalyzes the condensation between 1-deoxy-1-imino-d-erythrose 4-phosphate and phosphoenolpyruvate. RifA through rifE encode a type I polyketide synthase module, with the loading module being a non-ribosomal peptide synthase. In all, rifA-E assemble a linear undecaketide and are followed by rifF, which encodes an amide synthase and causes the undecaketide to release and form a macrolactam structure. Moreover, the rif cluster contains various regulatory proteins and glycosylating genes that appear to be silent. Other types of genes seem to perform post-synthase modifications of the original polyketide.

Derivatives
Lepetit introduced Rifampicin, an orally active rifamycin, around 1966. Rifabutin, a derivative of rifamycin S, was invented around 1975 and came on to the US market in 1993. Hoechst Marion Roussel (now part of AventisAventis
Aventis was a pharmaceutical and lab assay testing company. It was formed in 1999 when Rhône-Poulenc S.A. merged with Hoechst AG. The merged company was based in Strasbourg, France. With its headquarters in Strasbourg, Aventis was the product of the first transnational merger to combine large...
) introduced rifapentine in 1999.
Rifaximin is an oral rifamycin marketed in the US by Salix Pharmaceuticals that is not absorbed from the intestine. It is intended to treat intestinal infections due to Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
.
Currently available rifamycins
- RifampicinRifampicinRifampicin or rifampin is a bactericidal antibiotic drug of the rifamycin group. It is a semisynthetic compound derived from Amycolatopsis rifamycinica ...
or Rifampin - RifabutinRifabutinRifabutin is a bactericidal antibiotic drug primarily used in the treatment of tuberculosis. The drug is a semi-synthetic derivative of rifamycin S. Its effect is based on blocking the DNA-dependent RNA-polymerase of the bacteria. It is effective against Gram-positive and some Gram-negative...
- RifapentineRifapentineRifapentine is an antibiotic drug used in the treatment of tuberculosis.-History:...
- Rifaximin

