
Reuptake
Encyclopedia
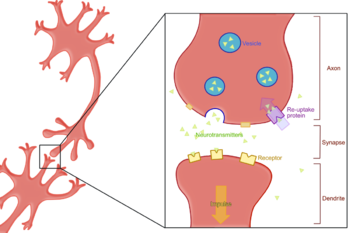
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
by a neurotransmitter transporter
Neurotransmitter transporter
Neurotransmitter transporters are a class of membrane transport proteins that span the cellular membranes of neurons. Their primary function is to carry neurotransmitters across these membranes and to direct their further transport to specific intracellular locations...
of a pre-synaptic
Synapse
In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another cell...
neuron
Neuron
A neuron is an electrically excitable cell that processes and transmits information by electrical and chemical signaling. Chemical signaling occurs via synapses, specialized connections with other cells. Neurons connect to each other to form networks. Neurons are the core components of the nervous...
after it has performed its function of transmitting a neural impulse
Action potential
In physiology, an action potential is a short-lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and...
.
Reuptake is necessary for normal synaptic physiology because it allows for the recycling of neurotransmitter
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
s and regulates the level of neurotransmitter present in the synapse and controls how long a signal resulting from neurotransmitter release lasts. Because neurotransmitters are too large and hydrophilic to diffuse through the membrane, specific transport proteins are necessary for the reabsorption of neurotransmitters. Much research, both biochemical and structural, has been performed to obtain clues about the mechanism of reuptake.
Protein structure
The 1st primary sequence of a reuptake protein was published in 1990. The technique for protein determination is contingent upon the purification, sequencing, and cloning of the transporter protein in question. After separate investigations had sequenced the DNA that coded for both GABAGamma-aminobutyric acid
γ-Aminobutyric acid is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays a role in regulating neuronal excitability throughout the nervous system...
transporter and norepinephrine
Norepinephrine
Norepinephrine is the US name for noradrenaline , a catecholamine with multiple roles including as a hormone and a neurotransmitter...
transporter, it could be seen that there were many similarities between the two DNA sequences. Further exploration in the field of reuptake proteins found that many of the transporters associated with important neurotransmitters within the body were also very similar in sequence to the GABA and norepinephrine transporters. The members of this new family include dopamine
Dopamine
Dopamine is a catecholamine neurotransmitter present in a wide variety of animals, including both vertebrates and invertebrates. In the brain, this substituted phenethylamine functions as a neurotransmitter, activating the five known types of dopamine receptors—D1, D2, D3, D4, and D5—and their...
, norepinephrine
Norepinephrine
Norepinephrine is the US name for noradrenaline , a catecholamine with multiple roles including as a hormone and a neurotransmitter...
, serotonin
Serotonin
Serotonin or 5-hydroxytryptamine is a monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal tract, platelets, and in the central nervous system of animals including humans...
, and GABA
Gamma-aminobutyric acid
γ-Aminobutyric acid is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays a role in regulating neuronal excitability throughout the nervous system...
. They were given the name Classical Na+/Cl- dependent transporters. Sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and Chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
ion dependence will be discussed later in the mechanism of action. Using the commonalities among sequences and hydropathy plot analyses, it was determined that there are 12 hydrophobic membrane spanning structural units in the ‘Classical’ transporter family. In addition to this, the Na and Cl termini exist in the intracellular space. These proteins also all have an extended fourth extracellular loop. There is an extracellular cavity in the protein, into which protrudes a helix hairpin formed by extracellular loop EL4. After using further more advanced scanning techniques, Masson et al. proposed that transmembrane unit 1 (closest to the Na-terminus) exists in the membrane as a pore loop. In other words, unit 1 would not exist in a transmembrane fashion but rather as a loop existing solely in the membrane. This model has been used before to explain mechanisms for ion selectivity filters, although the purpose of this pore loop is not readily apparent. This pore loop observation is consistent across all of the classical family of transporter proteins, suggesting that it is important for some function shared among them. Later experiments indicated that a tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
amino acid is highly conserved across transport proteins, and was shown to be essential for substrate binding and transport. Other important features include function specific positions in the transmembrane section 1, where an aspartate differentiates between monoamine substrates and a glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
differentiates between amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
substrates. It has also been shown that a negatively charged residue in the extracellular loop 5 and the transmembrane domain 10 may form an external gate.
Yamashita et al. chose to examine a bacterial homologue (LeuT: Leucine transporter) of these classical transporters from the bacterium Aquifex aeolicus
Aquifex aeolicus
Aquifex aeolicus is a rod-shaped prokaryote with a length of 2 to 6 micrometers and a diameter of around half a micrometer. It is one of a handful of species in the Aquificae phylum, an unusual group of thermophilic bacteria that are thought to be some of the oldest species of bacteria.A...
. Protein structure of LeuT was determined via crystallization. They chose to crystallize the system with leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
and its respective 2 sodium ions in order to visualize the pore and binding sites. The crystal was scanned via multi-wavelength anomalous dispersion
Multi-wavelength anomalous dispersion
Multi-wavelength anomalous diffraction is a technique used in X-ray crystallography that facilitates the determination of the three-dimensional structure of biological macromolecules via solution of the phase problem...
. Using this technique, they were able to replicate the findings in the classical transporters, but they determined that the folding and final structure were unique. They found that the transmembrane domains 1 and 6 contain unwound segments in the middle of the membrane. Along with this, transmembrane domains 3 and 8 and the areas surrounding the unwound sections of 1 and 6 form the substrate and sodium ion binding sites. They observed pseudo-symmetry among the LeuT protein, seen best when observing transmembrane domains 1-5 against 6-10.
Mechanism of action
The classic transporter proteins use symport to transport neurotransmitter across the membrane of the presynaptic neuron. The classical transporters, which are sodium and chloride ion dependent, take advantage of the large sodium gradients across the membrane. The neurotransmitter in question will bind to sodium ions, where the sodium ion will flow down its concentration gradient as well as electrical gradient. These forces will pull the neurotransmitter into the cell, against its own gradients. The chloride ion also contributes by flowing down its concentration gradient, but it flows against the electrical gradient, greatly reducing its efficiency as a symport ion. The role of the chloride ion in the symport mechanism is not exactly known, but has been implicated to be useful for counterbalancing the charge of the sodium symport ions. Because the neurotransmitter binds to sodium ions, their respective binding sites are very close in proximity. In the LeuT protein, the binding sites would be across transmembrane domains 3 and 8, and specifically the unwound sections of 1 and 6. These binding sites are made up of hydrogen and ionic bonding between the substrate and the transport protein. The primary sodium binding sites are Na1 and Na2, which exist in the transmembrane domains 1 (TM1) and 6 (TM6) in the unwound regions. In the LeuT example, 2 sodium ions bind to the substrate leucine. These 2 sodium ions work to pull leucine into the cell, but also by stabilizing the core of LeuT. Sodium ion 1 (binds to Na1) is octahedrally coordinated by the leucine carboxy oxygen, the carbonyl oxygens of Ala22 (transmembrane domain 1), Thr254 (TM6), side-chain carbonyl oxygens of Asn27 (TM1) and Asn286 (TM7), and the hydroxyl oxygen of Thr254 (TM6). The second sodium ion (binds to Na2) is trigonal bi-pyramidally coordinated by means of the carbonyl oxygens of Gly20 and Val23 (TM1), Ala351 (TM8), and the hydroxyl oxygens from Thr354 and Ser355 (TM8).After sodium ion coordination has taken place, some conformational change must occur. In an isolated system of TM3, TM8, TM1, and TM6, the unwound sections of 1 and 6 act like a joint and pivot the entire transmembrane domain relative to the rest of the protein and TM3 and TM8. The summation of actions mimics a half revolving door. Both TM3 and TM8 remain stationary and TM1 and TM6 swing about it.
The resulting system has affinity for both the substrate and sodium ions at both the intracellular level and the extracellular level. As the system progresses, the extracellular openings are blocked off by a gate. Because of the sodium gradient, it is unlikely that much substrate transfer occurs from the intracellular level to the extracellular level. The LeuT transporter protein is a homologue of the human classical transport proteins, and thus the proposed mechanism of action can be assumed to be largely similar to the human model.
Mechanism of reuptake inhibition
The main objective of a reuptake inhibitorReuptake inhibitor
A reuptake inhibitor , also known as a transporter blocker, is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron, leading to an increase in the extracellular concentrations of the neurotransmitter and therefore an...
is to substantially decrease the rate by which neurotransmitters are reabsorbed into the presynaptic neuron, leaving a net gain in the concentration of neurotransmitter in the synapse. This increases the probability and frequency of neurotransmitter binding to postsynaptic neurotransmitter receptors. Depending on the neurological system in question, a reuptake inhibitor can have drastic effects on cognition and behavior. Zhou et al. examined how tricyclic antidepressant
Tricyclic antidepressant
Tricyclic antidepressants are heterocyclic chemical compounds used primarily as antidepressants. The TCAs were first discovered in the early 1950s and were subsequently introduced later in the decade; they are named after their chemical structure, which contains three rings of atoms...
s act upon a reuptake protein in order to inhibit reuptake of the appropriate neurotransmitters. They chose to examine the LeuT system as it was previously crystallized by Yamashita et al. in 2005. They also chose to examine LeuT because it is a homologue of the transport proteins of concern when considering depression. They examined this relationship between tricyclic antidepressants and the LeuT system by crystallizing the two together, along with leucine and the respective 2 sodium ions. They found that the leucine and the sodium ions bound exactly as previously determined by Yamashita et al. They also observed that the tricyclic antidepressant (desipramine
Desipramine
Desipramine is a tricyclic antidepressant . It inhibits the reuptake of norepinephrine and to a lesser extent serotonin. It is used to treat depression, but not considered a first line treatment since the introduction of SSRI antidepressants...
) followed leucine into the pore. Desipramine effectively sits on top of leucine in LeuT and they both bind to the same protein residue. The tail of desipramine extends into the extracellular space. Most of the LeuT structure does not change, but the EL4 hairpin does form around desipramine as if it were locking it in place in the LeuT pore. Desipramine also acts by binding to the extracellular gate and forming a salt bridge between TM1 and TM10. This action prevents TM1 from pivoting, which prevents leucine from ever being exposed to the intracellular side of LeuT. Desipramine works as a reuptake inhibitor by effectively blocking and crowding up the pore of LeuT, which physically prevents its normal course of action.
Human systems
Horschitz et al. examined reuptake inhibitor selectivity among the rat serotonin reuptake protein (SERT) expressed in human embryonic kidney cells (HEK-SERT). They presented SERT with varying doses of either citalopram (SSRI, selective serotonin reuptake inhibitor) or desipramine (an inhibitor of norepinephrine reuptake protein, NET). By examining the dose-response curves (using a normal medium as control), they were able to quantify that citalopramCitalopram
Citalopram brand names: Celexa, Cipramil) is an antidepressant drug of the selective serotonin reuptake inhibitor class. It has U.S...
acted on SERT as an SSRI, and that desipramine had no effect on SERT. In a separate experiment, Horschitz et al. exposed HEK-SERT with citalopram on a long-term basis. They noticed that long-term exposure led to a down-regulation of binding sites. These results suggest some mechanism for long-term changes in the pre-synaptic neuron after drug therapy. Horschitz et al. found that after removing citalopram from the system, normal levels of SERT binding site expression returned.
Depression has been suggested to be a result of a decrease of serotonin found in the synapse. This theory has been supported by the successful reduction of depressive symptoms after administration of tri-cyclic antidepressants (such as desipramine) and SSRI’s. Tri-cyclic antidepressants inhibit the reuptake of both serotonin and norepinephrine by acting upon both the SERT and NET. SSRIs selectively inhibit the reuptake of serotonin by acting upon SERT. The net result is an increased amount of serotonin in the synapse, thus increasing the probability that serotonin will interact with a serotonin receptor of the postsynaptic neuron. There are additional mechanisms by which serotonin autoreceptor desensitization must occur, but the net result is the same. This increases serotonin signaling, which then acts to elevate mood and thus relieve depressive symptoms.
The net effect of amphetamine
Amphetamine
Amphetamine or amfetamine is a psychostimulant drug of the phenethylamine class which produces increased wakefulness and focus in association with decreased fatigue and appetite.Brand names of medications that contain, or metabolize into, amphetamine include Adderall, Dexedrine, Dextrostat,...
(AMPH) use is an increase of dopamine in the synapse. It has been shown that AMPH acts upon the dopamine reuptake protein (DAT) in a reverse fashion. In DAT knockout mice
Knockout mouse
A knockout mouse is a genetically engineered mouse in which researchers have inactivated, or "knocked out," an existing gene by replacing it or disrupting it with an artificial piece of DNA...
, dopamine levels in the synapse (as measured by microdialysis) were no different when they were exposed to AMPH relative to baseline levels. In normal mice, levels of dopamine in the synapse rose to ten times normal levels after exposure to AMPH.

