Relative volatility
Encyclopedia
Relative volatility is a measure comparing the vapor pressure
s of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation
processes. In effect, it indicates the ease or difficulty of using distillation to separate the more volatile components from the less volatile components in a mixture. By convention, relative volatility is usually denoted as .
.
Relative volatilities are used in the design of all types of distillation processes as well as other separation
or absorption processes that involve the contacting of vapor
and liquid
phases in a series of equilibrium stages.
Relative volatilities are not used in separation or absorption processes that involve components reacting
with each other (for example, the absorption of gaseous carbon dioxide
in aqueous solutions of sodium hydroxide).
and pressure
, the relative volatility is defined as
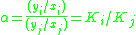
When their liquid concentrations are equal, more volatile components have higher vapor pressures than less volatile components. Thus, a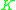 value (=
value (= 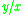 ) for a more volatile component is larger than a
) for a more volatile component is larger than a  value for a less volatile component. That means that
value for a less volatile component. That means that  ≥ 1 since the larger
≥ 1 since the larger  value of the more volatile component is in the numerator and the smaller
value of the more volatile component is in the numerator and the smaller  of the less volatile component is in the denominator.
of the less volatile component is in the denominator.
 is a unitless quantity. When the volatilities of both key components are equal,
is a unitless quantity. When the volatilities of both key components are equal,  = 1 and separation of the two by distillation would be impossible under the given conditions because the compositions of the liquid and the vapor phase are the same (azeotrope
= 1 and separation of the two by distillation would be impossible under the given conditions because the compositions of the liquid and the vapor phase are the same (azeotrope
). As the value of increases above 1, separation by distillation becomes progressively easier.
increases above 1, separation by distillation becomes progressively easier.
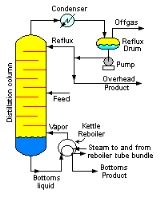
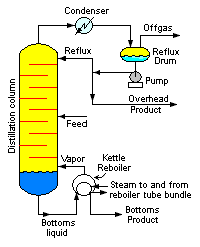 A liquid mixture containing two components is called a binary mixture. When a binary mixture is distilled, complete separation of the two components is rarely achieved. Typically, the overhead fraction from the distillation column consists predominantly of the more volatile component and some small amount of the less volatile component and the bottoms fraction consists predominantly of the less volatile component and some small amount of the more volatile component.
A liquid mixture containing two components is called a binary mixture. When a binary mixture is distilled, complete separation of the two components is rarely achieved. Typically, the overhead fraction from the distillation column consists predominantly of the more volatile component and some small amount of the less volatile component and the bottoms fraction consists predominantly of the less volatile component and some small amount of the more volatile component.
A liquid mixture containing many components is called a multi-component mixture. When a multi-component mixture is distilled, the overhead fraction and the bottoms fraction typically contain much more than one or two components. For example, some intermediate products in an oil refinery
are multi-component liquid mixtures that may contain the alkane
, alkene
and alkyne
hydrocarbon
s ranging from methane
having one carbon
atom
to decane
s having ten carbon atoms. For distilling such a mixture, the distillation column may be designed (for example) to produce:
Such a distillation column is typically called a depropanizer. The designer would designate the key components governing the separation design to be propane as the so-called light key (LK) and isobutane as the so-called heavy key (HK). In that context, a lighter component means a component with a lower boiling point
(or a higher vapor pressure) and a heavier component means a component with a higher boiling point (or a lower vapor pressure).
Thus, for the distillation of any multi-component mixture, the relative volatility is often defined as
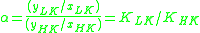
Large-scale industrial distillation is rarely undertaken if the relative volatility is less than 1.05.
The values of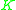 have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known DePriester charts.
have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known DePriester charts.
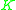 values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in oil refineries, petrochemical
values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in oil refineries, petrochemical
and chemical plant
s, natural gas processing
plants and other industries.
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
s of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
processes. In effect, it indicates the ease or difficulty of using distillation to separate the more volatile components from the less volatile components in a mixture. By convention, relative volatility is usually denoted as
 .
.Relative volatilities are used in the design of all types of distillation processes as well as other separation
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
or absorption processes that involve the contacting of vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
and liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
phases in a series of equilibrium stages.
Relative volatilities are not used in separation or absorption processes that involve components reacting
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
with each other (for example, the absorption of gaseous carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
in aqueous solutions of sodium hydroxide).
Definition
For a liquid mixture of two components (called a binary mixture) at a given temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, the relative volatility is defined as
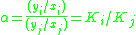
| where: | |
 |
= the relative volatility of the more volatile component 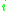 to the less volatile component to the less volatile component 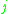 |
|---|---|
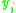 |
= the vapor-liquid equilibrium Vapor-liquid equilibrium Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion... concentration of component 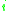 in the vapor phase in the vapor phase |
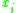 |
= the vapor-liquid equilibrium concentration of component 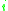 in the liquid phase in the liquid phase |
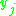 |
= the vapor-liquid equilibrium concentration of component 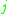 in the vapor phase in the vapor phase |
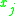 |
= the vapor-liquid equilibrium concentration of component 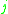 in the liquid phase in the liquid phase |
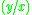 |
= 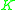 commonly called the K value or vapor-liquid distribution ratio of a component commonly called the K value or vapor-liquid distribution ratio of a component |
When their liquid concentrations are equal, more volatile components have higher vapor pressures than less volatile components. Thus, a
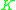 value (=
value (= 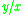 ) for a more volatile component is larger than a
) for a more volatile component is larger than a 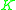 value for a less volatile component. That means that
value for a less volatile component. That means that  ≥ 1 since the larger
≥ 1 since the larger 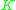 value of the more volatile component is in the numerator and the smaller
value of the more volatile component is in the numerator and the smaller 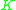 of the less volatile component is in the denominator.
of the less volatile component is in the denominator. is a unitless quantity. When the volatilities of both key components are equal,
is a unitless quantity. When the volatilities of both key components are equal,  = 1 and separation of the two by distillation would be impossible under the given conditions because the compositions of the liquid and the vapor phase are the same (azeotrope
= 1 and separation of the two by distillation would be impossible under the given conditions because the compositions of the liquid and the vapor phase are the same (azeotropeAzeotrope
An azeotrope is a mixture of two or more liquids in such a ratio that its composition cannot be changed by simple distillation. This occurs because, when an azeotrope is boiled, the resulting vapor has the same ratio of constituents as the original mixture....
). As the value of
 increases above 1, separation by distillation becomes progressively easier.
increases above 1, separation by distillation becomes progressively easier.
A liquid mixture containing many components is called a multi-component mixture. When a multi-component mixture is distilled, the overhead fraction and the bottoms fraction typically contain much more than one or two components. For example, some intermediate products in an oil refinery
Oil refinery
An oil refinery or petroleum refinery is an industrial process plant where crude oil is processed and refined into more useful petroleum products, such as gasoline, diesel fuel, asphalt base, heating oil, kerosene, and liquefied petroleum gas...
are multi-component liquid mixtures that may contain the alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
, alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s ranging from methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
having one carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
to decane
Decane
Decane is an alkane hydrocarbon with the chemical formula CH38CH3.75 structural isomers of decane exist, all of which are flammable liquids. Decane is one of the components of gasoline . Like other alkanes, it is nonpolar and therefore will not dissolve in polar liquids such as water...
s having ten carbon atoms. For distilling such a mixture, the distillation column may be designed (for example) to produce:
- An overhead fraction containing predominantly the more volatile components ranging from methane (having one carbon atom) to propanePropanePropane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central...
(having three carbon atoms) - A bottoms fraction containing predominantly the less volatile components ranging from isobutaneIsobutaneIsobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,...
(having four carbon atoms) to decanes (ten carbon atoms).
Such a distillation column is typically called a depropanizer. The designer would designate the key components governing the separation design to be propane as the so-called light key (LK) and isobutane as the so-called heavy key (HK). In that context, a lighter component means a component with a lower boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
(or a higher vapor pressure) and a heavier component means a component with a higher boiling point (or a lower vapor pressure).
Thus, for the distillation of any multi-component mixture, the relative volatility is often defined as
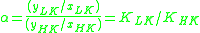
Large-scale industrial distillation is rarely undertaken if the relative volatility is less than 1.05.
The values of
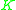 have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known DePriester charts.
have been correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the well-known DePriester charts.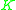 values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in oil refineries, petrochemical
values are widely used in the design of large-scale distillation columns for distilling multi-component mixtures in oil refineries, petrochemicalPetrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
and chemical plant
Chemical plant
A chemical plant is an industrial process plant that manufactures chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transformation and or separation of materials. Chemical plants use special equipment,...
s, natural gas processing
Natural gas processing
Natural-gas processing is a complex industrial process designed to clean raw natural gas by separating impurities and various non-methane hydrocarbons and fluids to produce what is known as pipeline quality dry natural gas.-Background:...
plants and other industries.
See also
- Continuous distillationContinuous distillationContinuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. A distillation is the separation or partial separation of a liquid feed mixture into components or...
- Fractional distillationFractional distillationFractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
- Vacuum distillationVacuum distillationVacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor pressure causing evaporation of the most volatile liquid...
- Fractionation column
- Theoretical plateTheoretical plateA theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
- McCabe-Thiele methodMcCabe-Thiele methodThe McCabe-Thiele method was presented by two graduate students at Massachusetts Institute of Technology , Warren L. McCabe and Ernest W. Thiele in 1925. The technique is considered to be the simplest and perhaps most instructive method for analysis of binary distillation...
- Fenske equationFenske equationThe Fenske equation in continuous fractional distillation is an equation used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a fractionation column that is being operated at total reflux .The equation was derived by Merrell Fenske in...
- Equilibrium flash of a multi-component liquid
- Volatility (chemistry)Volatility (chemistry)In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
External links
- Distillation Theory by Ivar J. Halvorsen and Sigurd Skogestad, Norwegian University of Science and TechnologyNorwegian University of Science and TechnologyThe Norwegian University of Science and Technology , commonly known as NTNU, is located in Trondheim. NTNU is the second largest of the eight universities in Norway, and, as its name suggests, has the main national responsibility for higher education in engineering and technology...
(scroll down to: 2.2.3 K-values and Relative Volatility)
- Distillation Principals by Ming T. Tham, University of Newcastle upon TyneUniversity of Newcastle upon TyneNewcastle University is a major research-intensive university located in Newcastle upon Tyne in the north-east of England. It was established as a School of Medicine and Surgery in 1834 and became the University of Newcastle upon Tyne by an Act of Parliament in August 1963. Newcastle University is...
(scroll down to Relative Volatility)

