
Recrystallization (chemistry)
Encyclopedia
Chemistry
In chemistryChemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, recrystallization is a procedure for purifying compounds
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted (see Separation process
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
), which includes recrystallization. There are also different recrystallization techniques that can be used such as:
Single-solvent recrystallization
Typically, the mixture of "compound A" and "impurity B" are dissolved in the smallest amount of hot solvent to fully dissolve the mixture, thus making a saturatedSaturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
. The solution is then allowed to cool. As the solution cools the solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
of compounds in solution drops. This results in the desired compound dropping (recrystallizing) from solution. The slower the rate of cooling, the bigger the crystals formed.
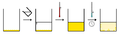
Filtration
Filtration is commonly the mechanical or physical operation which is used for the separation of solids from fluids by interposing a medium through which only the fluid can pass...
and the filtrate is discarded. If the solubility product of the impurity is exceeded, some of the impurity will co-precipitate. However, because of the relatively low concentration of the impurity, its concentration in the precipitated crystals will be less than its concentration in the original solid. Repeated recrystallization will result in an ever purer crystalline precipitate. The purity is checked after each recrystallization by measuring the melting point, since impurities lower the melting point
Melting point depression
Melting-point depression is a term referring to the phenomenon of reduction of the melting point of a material with reduction of its size. This phenomenon is very prominent in nanoscale materials which melt at temperatures hundreds of degrees lower than bulk materials.-Introduction:The melting...
. NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
can also be used to check the level of impurity. Repeated recrystallization results in some loss of material because of the finite solubility of compound A.
The crystallization process requires an initiation step, such as the addition of a "seed" crystal. In the laboratory a minuscule fragment of glass, produced by scratching the side of the glass recrystallization vessel, may provide the nucleus on which crystals may grow.
Successful recrystallization depends on finding the right solvent. This is usually a combination of prediction/experience and trial/error. The compounds must be more soluble at the higher temperature than at the lower temperatures. Any insoluble impurity is removed by the technique of hot filtration
Funnels (laboratory)
Laboratory funnels are funnels that have been made for use in the chemical laboratory. There are many different kinds of funnels that have been adapted for these specialized applications. Filter funnels, thistle funnels , and dropping funnels have stopcocks which allow the fluids to be added to a...
.
Multi-solvent recrystallization
This method is the same as the above but where two (or more) solvents are used. This relies on both "compound A" and "impurity B" being soluble in a first solvent. A second solvent is slowly added. Either "compound A" or "impurity B" will be insoluble in this solvent and precipitate, whilst the other of "compound A"/"impurity B" will remain in solution. Thus the proportion of first and second solvents is critical. Typically the second solvent is added slowly until one of the compounds begins to crystallize from solution and then the solution is cooled. Heating is not required for this technique but can be used.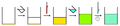

Hot filtration-recrystallization
Hot filtration can be used to separate "compound A" from both "impurity B" and some "insoluble matter C". This technique normally uses a single-solvent system as described above. When both "compound A" and "impurity B" are dissolved in the minimum amount of hot solvent, the solution is filtered to remove "insoluble matter C". This matter may be anything from a third impurity compound to fragments of broken glass. For a successful procedure, one must ensure that the filtration apparatus is hot in order to stop the dissolved compounds crystallizing from solution during filtration, thus forming crystals on the filter paper or funnel.One way to achieve this is to heat a conical flask containing a small amount of clean solvent on a hot plate. A filter funnel is rested on the mouth, and hot solvent vapors keep the stem warm. Jacketed filter funnels may also be used. The filter paper is preferably fluted, rather than folded into a quarter; this allows quicker filtration, thus less opportunity for the desired compound to cool and crystallize from the solution.
Often it is simpler to do the filtration and recrystallization as two independent and separate steps. That is dissolve "compound A" and "impurity B" in a suitable solvent at room temperature, filter (to remove insoluble compound/glass), remove the solvent and then recrystallize using any of the methods listed above.

Seeding
CrystallizationCrystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
requires an initiation step. This can be spontaneous or can be done by adding a small amount of the pure compound (a seed crystal
Seed crystal
A seed crystal is a small piece of single crystal/polycrystal material from which a large crystal of the same material typically is to be grown...
) to the saturated solution, or can be done by simply scratching the glass surface to create a seeding surface for crystal growth
Crystal growth
A crystal is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists in the addition of new atoms, ions, or polymer strings into...
. It is thought that even dust particles can act as simple seeds.
Single perfect crystals (for X-ray analysis)
Growing crystals for X-ray crystallographyX-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
can be quite difficult. For X-ray analysis, single perfect crystals are required. Typically a small amount (5–100 mg) of pure compound is used, and crystals are allowed to grow very slowly. Several techniques can be used to grow these perfect crystals:
- Slow evaporation of a single solvent - typically the compound is dissolved in a suitable solvent and the solvent is allowed to slowly evaporate. Once the solution is saturated crystals can form.
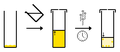
- Slow evaporation of a multi-solvent system - the same as above, however as the solvent composition changes due to evaporation of the more volatile solvent. The compound is more soluble in the volatile solvent, and so the compound becomes increasingly insoluble in solution and crystallizes.
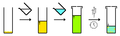
- Slow diffusion - similar to the above. However, a second solvent is allowed to evaporate from one container into a container holding the compound solution (gas-diffusion). As the solvent composition changes due to an increase in solvent that has gas-diffused into solution, the compound become increasingly insoluble in solution and crystallizes.

- Interface/slow mixing (often performed in an NMR tubeNMR tubeAn NMR tube is a thin glass walled tube used to contain samples in nuclear magnetic resonance spectroscopy. Typically NMR tubes come in 5 mm diameters but 10 mm and 3 mm samples are known. It is important that the tubes are uniformly thick and well-balanced to ensure that NMR tube...
). Similar to the above, but instead of one solvent gas-diffusing into another, the two solvents mix (diffuse) by liquid-liquid diffusion. Typically a second solvent is "layered" carefully on top of the solution containing the compound. Over time the two solution mix. As the solvent composition changes due to diffusion, the compound becomes increasingly insoluble in solution and crystallizes, usually at the interface.

- Specialized equipment can be used in the shape of a "H" to perform the above, where one of the vertical line of the "H" is a tube containing a solution of the compound, and the other vertical line of the "H" is a tube containing a solvent which the compound is not soluble in, and the horizontal line of the "H" is a tube which joins the two vertical tubes, which also has a fine glass sinter that restricts the mixing of the two solvents.

- Once single perfect crystals have been obtained, it is recommended that the crystals are kept in a sealed vessel with some of the liquid of crystallisation to prevent the crystal from 'drying out'. Single perfect crystals may contain solvent of crystallisation in the crystal lattice. Loss of this internal solvent from the crystals can result in the crystal lattice breaking down, and the crystals turning to powder.
Ice
For iceIce
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
, recrystallization refers to the growth of larger crystals at the expense of smaller ones. Some biological antifreeze protein
Antifreeze protein
Antifreeze proteins or ice structuring proteins refer to a class of polypeptides produced by certain vertebrates, plants, fungi and bacteria that permit their survival in subzero environments. AFPs bind to small ice crystals to inhibit growth and recrystallization of ice that would otherwise be...
s have been shown to inhibit this process, and the effect may be relevant in freezing-tolerant organisms.
See also
- Crystal structureCrystal structureIn mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
- CrystallizationCrystallizationCrystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
and engineering aspects - Fractional crystallization (chemistry)Fractional crystallization (chemistry)In chemistry, fractional crystallization is a method of refining substances based on differences in solubility. If a mixture of two or more substances in solution is allowed to crystallize, for example by allowing the temperature of the solution to decrease, the precipitate will contain more of...
- Laser-heated pedestal growthLaser-heated pedestal growthLaser-heated pedestal growth is a crystal growth technique. The technique can be viewed as a miniature floating zone, where the heat source is replaced by a powerful CO2 or YAG laser...
- Seed crystalSeed crystalA seed crystal is a small piece of single crystal/polycrystal material from which a large crystal of the same material typically is to be grown...
- Single crystalSingle crystalA single crystal or monocrystalline solid is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries...

