
Reactivity
Encyclopedia
Reactivity in chemistry refers to
The chemical reactivity of a single substance (reactant) covers its behaviour in which
The chemical reactivity of a substance can refer to
A responsible discussion of chemical reactivity can be found in any standard textbook on physical chemistry.
Restriction of the term to refer to reaction rates leads to a more consistent view. Reactivity then refers to the rate
at which a chemical substance
tends to undergo a chemical reaction
in time. In pure compound
s, reactivity is regulated by the physical properties of the sample. For instance, grinding a sample to a higher specific surface area increases its reactivity. In impure compounds, the reactivity is also affected by the inclusion of contaminants. In crystalline compounds, the crystalline form can also affect reactivity. However in all cases, reactivity is primarily due to the sub-atomic properties of the compound.
Although it is commonplace to make statements that substance 'X is reactive', all substances react with some reagents and not others. For example, in making the statement that 'sodium metal is reactive', we are alluding to the fact that sodium reacts with many common reagents (including pure oxygen, chlorine, hydrochloric acid, water) and/or that it reacts rapidly with such materials at either room temperature or using a bunsen flame.
'Stability' should not be confused with reactivity. For example, an isolated molecule of an electronically state of the oxygen molecule will spontaneously emit light after a statistically defined period. The half-life of such a species is another manifestation of its stability, but its reactivity can only be ascertained via its reactions with other species.
occurs because the products (taken as a group) are at a lower free energy
than the reactants; the lower energy state is referred to as the 'more stable state'. Quantum chemistry
provides the most in-depth and exact understanding of the reason this occurs. Generally, electron
s exist in orbital
s that are the result of solving the Schrödinger equation
for specific situations.
All things (values of the n and ml quantum numbers) being equal, the order of stability of electrons in a system from least to greatest is unpaired with no other electrons in similar orbitals, unpaired with all degenerate orbitals half filled and the most stable is a filled set of orbitals. In order to achieve one of these orders of stability, an atom will react with another atom, thereby stabilizing both atoms. For example, a lone hydrogen
atom has a single electron in its 1s orbital. It becomes significantly more stable (as much as 100 kilocalories
per mole
, or 420 kilojoules
per mole) when reacting to form H2.
It is for this same reason that carbon
will almost always form four bonds
. Its ground state valence
configuration is 2s2 2p2, half filled. However, the activation energy
to go from half filled to fully filled p orbitals is so small it is negligible, and as such carbon will form them almost instantaneously, meanwhile the process releases a significant amount of energy (exothermic
). This four equal bond configuration is called sp3 hybridization
.
The above three paragraphs rationalise, albeit very generally, the reactions of some common species, particularly atoms, but chemists have so far been unable to jump from such general considerations to quantitative models of reactivity.
Reactants → Products
is governed by the rate law:
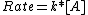
where the rate
is the change in the molar concentration in one second in the rate-determining step of the reaction (the slowest step), [A] is the product of the molar concentration of all the reactants raised to the correct order, known as the reaction order, and k is the reaction constant, which is constant for one given set of circumstances (generally temperature and pressure) and independent of concentration. The greater the reactivity of a compound the higher the value of k and the higher the rate. For instance, if,
A+B → C+D
Then:

where n is the reaction order of A, m is the reaction order of B, n+m is the reaction order of the full reaction, and k is the reaction constant.
- the chemical reactions of a single substance,
- the chemical reactions of two or more substances that interact with each other,
- the systematic study of sets of reactions of these two kinds,
- methodology that applies to the study of reactivity of chemicals of all kinds,
- experimental methods that are used to observe these processes,
- theories to predict and to account for these processes.
The chemical reactivity of a single substance (reactant) covers its behaviour in which
- it decomposes,
- it forms new substances by addition of atoms from another reactant or reactants,
- reactions in which it interacts with two or more other reactants to form two or more products.
The chemical reactivity of a substance can refer to
- the variety of circumstances (conditions that include temperature, pressure, presence of catalysts) in which it reacts, in combination with
- the variety of substances with which it reacts,
- the equilibrium point of the reaction (i.e. the extent to which all of it reacts),
- the rate of the reaction.
A responsible discussion of chemical reactivity can be found in any standard textbook on physical chemistry.
An alternative point of view
Reactivity is a somewhat vague concept used in chemistry which appears to embody both thermodynamic factors and kinetic factors i.e. 'whether or not a substance reacts and how fast it reacts'. Both factors are actually distinct and both are commonly temperature dependent. For example, it is commonly asserted that the reactivity of group one metals (Na, K, etc) increases down the group in the periodic table, or that hydrogen's reactivity is evidenced by its reaction with oxygen. In fact, the rate of reaction of alkali metals (as evidenced by their reaction with water for example) is a function not only of position within the group but particle size; and hydrogen will not react with oxygen even though the equilibrium constant is very large unless a flame initiates the radical reaction which leads to an explosion.Restriction of the term to refer to reaction rates leads to a more consistent view. Reactivity then refers to the rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
at which a chemical substance
Chemical substance
In chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e. without breaking chemical bonds. They can be solids, liquids or gases.Chemical substances are...
tends to undergo a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
in time. In pure compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s, reactivity is regulated by the physical properties of the sample. For instance, grinding a sample to a higher specific surface area increases its reactivity. In impure compounds, the reactivity is also affected by the inclusion of contaminants. In crystalline compounds, the crystalline form can also affect reactivity. However in all cases, reactivity is primarily due to the sub-atomic properties of the compound.
Although it is commonplace to make statements that substance 'X is reactive', all substances react with some reagents and not others. For example, in making the statement that 'sodium metal is reactive', we are alluding to the fact that sodium reacts with many common reagents (including pure oxygen, chlorine, hydrochloric acid, water) and/or that it reacts rapidly with such materials at either room temperature or using a bunsen flame.
'Stability' should not be confused with reactivity. For example, an isolated molecule of an electronically state of the oxygen molecule will spontaneously emit light after a statistically defined period. The half-life of such a species is another manifestation of its stability, but its reactivity can only be ascertained via its reactions with other species.
Causes of reactivity
The second meaning of 'reactivity', that of whether or not a substance reacts, can be rationalised at the atomic and molecular level using older and simpler valence bond theory and also atomic and molecular orbital theory. Thermodynamically, a chemical reactionChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
occurs because the products (taken as a group) are at a lower free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
than the reactants; the lower energy state is referred to as the 'more stable state'. Quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
provides the most in-depth and exact understanding of the reason this occurs. Generally, electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s exist in orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s that are the result of solving the Schrödinger equation
Schrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
for specific situations.
All things (values of the n and ml quantum numbers) being equal, the order of stability of electrons in a system from least to greatest is unpaired with no other electrons in similar orbitals, unpaired with all degenerate orbitals half filled and the most stable is a filled set of orbitals. In order to achieve one of these orders of stability, an atom will react with another atom, thereby stabilizing both atoms. For example, a lone hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom has a single electron in its 1s orbital. It becomes significantly more stable (as much as 100 kilocalories
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
per mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
, or 420 kilojoules
Joule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
per mole) when reacting to form H2.
It is for this same reason that carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
will almost always form four bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
. Its ground state valence
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
configuration is 2s2 2p2, half filled. However, the activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
to go from half filled to fully filled p orbitals is so small it is negligible, and as such carbon will form them almost instantaneously, meanwhile the process releases a significant amount of energy (exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
). This four equal bond configuration is called sp3 hybridization
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
.
The above three paragraphs rationalise, albeit very generally, the reactions of some common species, particularly atoms, but chemists have so far been unable to jump from such general considerations to quantitative models of reactivity.
Chemical kinetics: reaction rate as reactivity
The rate of any given reaction,Reactants → Products
is governed by the rate law:
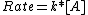
where the rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
is the change in the molar concentration in one second in the rate-determining step of the reaction (the slowest step), [A] is the product of the molar concentration of all the reactants raised to the correct order, known as the reaction order, and k is the reaction constant, which is constant for one given set of circumstances (generally temperature and pressure) and independent of concentration. The greater the reactivity of a compound the higher the value of k and the higher the rate. For instance, if,
A+B → C+D
Then:

where n is the reaction order of A, m is the reaction order of B, n+m is the reaction order of the full reaction, and k is the reaction constant.
See also
- catalysisCatalysisCatalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
- Reactivity seriesReactivity seriesIn introductory chemistry, the reactivity series or activity series is an empirical series of metals, in order of "reactivity" from highest to lowest...
- Michaelis-Menten equation
- organic chemistryOrganic chemistryOrganic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
- chemical kineticsChemical kineticsChemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...

