
Pyranose
Encyclopedia
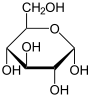
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle pyran
Pyran
In chemistry, a pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the location of the double bonds...
, but the pyranose ring does not have double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s. A pyranose in which the anomeric OH at C(l) has been converted into an OR group is called a pyranoside.
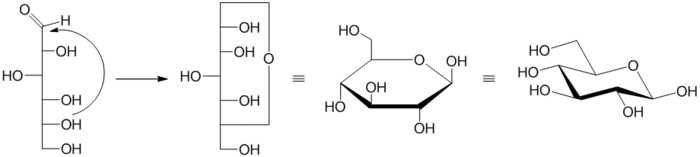
Formation of Pyranose
The pyranose ring is formed by the reaction of the hydroxylHydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group on carbon 5 (C-5) of a sugar with the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
at carbon 1. This forms an intramolecular hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
. If reaction is between the C-4 hydroxyl and the aldehyde, a furanose
Furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom...
is formed instead. The pyranose form is thermodynamically more stable than the furanose form, which can be seen by the distribution of these two cyclic forms in solution.
History

Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
won the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
(1902) for his work in determining the structure of the D-aldohexose
Aldohexose
An aldohexose is a hexose with an aldehyde group on one end.The aldohexoses have four chiral centres for a total of 16 possible aldohexose stereoisomers . Of these, only three commonly occur in nature: D-glucose, D-galactose, and D-mannose...
s. However, the linear, free-aldehyde structures that Fischer proposed represent a very minor percentage of the forms that hexose sugars adopt in solution. It was Edmund Hirst
Edmund Hirst
Sir Edmund Langley Hirst CBE FRS FRSE , was a British chemist.He held the Forbes Chair of Organic Chemistry at Edinburgh University and was head of department there from 1959 to 1964....
and Clifford Purves, in the research group of Walter Haworth
Walter Haworth
Sir Norman Haworth was a British chemist best known for his groundbreaking work on ascorbic acid while working at the University of Birmingham. He received the 1937 Nobel Prize in Chemistry "for his investigations on carbohydrates and vitamin C"...
, who conclusively determined that the hexose sugars preferentially form a pyranose, or six-membered, ring. Haworth drew the ring as a flat hexagon with groups above and below the plane of the ring – the Haworth projection
Haworth projection
A Haworth projection is a common way of representing the cyclic structure of monosaccharides with a simple three-dimensional perspective.The Haworth projection was named after the English chemist Sir Norman Haworth....
.
A further refinement to the conformation of pyranose rings came when Sponsler and Dore (1926) realized that Sachse’s mathematical treatment of six-membered rings could be applied to their X-ray structure
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
of cellulose
Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to over ten thousand β linked D-glucose units....
. It was determined that the pyranose ring is puckered, to allow all of the carbon atoms of the ring to have close to the ideal tetrahedral geometry.
Conformation of the Pyranose Ring
This puckering leads to a total of 38 distinct basic pyranose conformationsConformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
: 2 chairs, 6 boats, 6 skew-boats, 12 half-chairs, and 12 envelopes.
.png)

Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
to interconversion may be present. The energy of these conformations can be calculated from quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
; an example of possible glucopyranose interconversions is given.
The conformations of the pyranose ring are superficially similar to that of the cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
ring. However, the specific nomenclature of pyranoses includes reference to the ring oxygen, and the presence of hydroxyls on the ring have distinct effects on its conformational preference. There are also conformational and stereochemical effects specific to the pyranose ring.
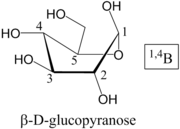
Nomenclature of Pyranose Rings
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
, and these form the basis of the name. Common conformations are Chair (C), Boat (B), Skew (S), Half-Chair (H) or Envelope (E). The ring atoms are then numbered; the anomer
Anomer
In carbohydrate chemistry, an anomer is a special type of epimer. It is one of two stereoisomers of a cyclic saccharide that differs only in its configuration at the hemiacetal or hemiketal carbon, also called the anomeric carbon. Anomerization is the process of conversion of one anomer to the other...
ic, or hemiacetal, carbon is always 1. Oxygen atoms in the structure are, in general, referred to by the carbon atom they are attached to in the acyclic form, and designated O. Then:
- Position the ring so that, if looking at the top face, the atoms are numbered clockwise. 4 (or 5, in the case of an envelope) atoms will be in a plane
- Atoms above the plane are written before the conformer label, as a superscript
- Atoms below the plane are written following the conformer label, as a subscript
NMR Spectroscopy of Pyranose Rings
As shown by the relative structure energies in the diagram above, the chair structures are the most stable carbohydrate form. This relatively defined and stable conformation means that the hydrogen atoms of the pyranose ring are held at relatively constant angles to one another. Carbohydrate NMRProton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
takes advantage of these dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s to determine the configuration of each of the hydroxyl groups around the ring.

