
Proton exchange membrane fuel cell
Encyclopedia
Proton exchange membrane
A proton exchange membrane or polymer electrolyte membrane is a semipermeable membrane generally made from ionomers and designed to conduct protons while being impermeable to gases such as oxygen or hydrogen...
fuel cells, also known as polymer electrolyte membrane (PEM) fuel cells (PEMFC), are a type of fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
being developed for transport applications as well as for stationary fuel cell applications and portable fuel cell applications
Portable fuel cell applications
Fuel cell applications are stationary fuel cell applications and portable fuel cell plications...
. Their distinguishing features include lower temperature/pressure ranges (50 to 100 °C) and a special polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
membrane
Artificial membrane
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of twentieth century. A...
. They are a leading candidate to replace the aging alkaline fuel cell technology, which was used in the Space Shuttle
Space Shuttle
The Space Shuttle was a manned orbital rocket and spacecraft system operated by NASA on 135 missions from 1981 to 2011. The system combined rocket launch, orbital spacecraft, and re-entry spaceplane with modular add-ons...
.
Reactions
A proton exchange membrane fuel cell transforms the chemical energyChemical energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances...
liberated during the electrochemical reaction of hydrogen and oxygen to electrical energy, as opposed to the direct combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of hydrogen and oxygen gases to produce thermal energy
Thermal energy
Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature....
.
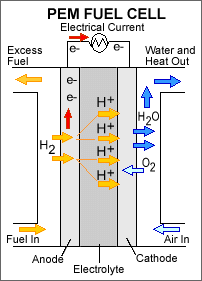
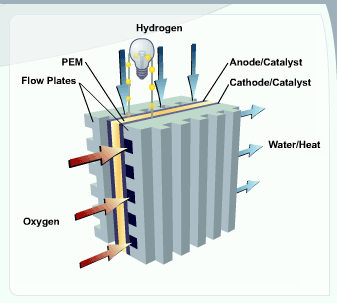
A stream of hydrogen is delivered to the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
side of the membrane electrode assembly
Membrane electrode assembly
A membrane electrode assembly is an assembled stack of proton exchange membranes or alkali anion exchange membrane , catalyst and flat plate electrode used in a fuel cell.-PEM-MEA:...
(MEA). At the anode side it is catalytically
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
split into proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s and electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s. This oxidation half-cell reaction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
is represented by:
At the Anode:
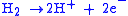 |
 SHE SHEStandard hydrogen electrode The standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials... |
 |
The newly formed protons permeate through the polymer electrolyte membrane to the cathode side. The electrons travel along an external load circuit
Electrical network
An electrical network is an interconnection of electrical elements such as resistors, inductors, capacitors, transmission lines, voltage sources, current sources and switches. An electrical circuit is a special type of network, one that has a closed loop giving a return path for the current...
to the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
side of the MEA, thus creating the current output of the fuel cell.
Meanwhile, a stream of oxygen is delivered to the cathode side of the MEA. At the cathode side oxygen molecules react with the protons permeating through the polymer electrolyte membrane and the electrons arriving through the external circuit to form water molecules. This reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
half-cell reaction is represented by:
At the Cathode:
 |
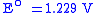 SHE SHEStandard hydrogen electrode The standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials... |
 |
Overall reaction:
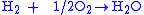 |
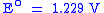 SHE SHEStandard hydrogen electrode The standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials... |
 |
Polymer electrolyte membrane
Short circuit
A short circuit in an electrical circuit that allows a current to travel along an unintended path, often where essentially no electrical impedance is encountered....
" the fuel cell. The membrane must also not allow either gas to pass to the other side of the cell, a problem known as gas crossover. Finally, the membrane must be resistant to the reducing environment at the cathode as well as the harsh oxidative environment at the anode.
Splitting of the hydrogen molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
is relatively easy by using a platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
catalyst. Unfortunately however, splitting the oxygen molecule is more difficult, and this causes significant electric losses. An appropriate catalyst material for this process has not been discovered, and platinum is the best option. One promising catalyst that uses far less expensive materials—iron, nitrogen, and carbon—has long been known to promote the necessary reactions, but at rates that are far too slow to be practical. Recently researchers at the Institut National de la Recherche Scientifique (INRS) in Quebec have dramatically increased the performance of this type of iron-based catalyst. Their material produces 99 amperes per cubic centimeter at 0.8 volts, a key measurement of catalytic activity . That is 35 times better than the best nonprecious metal catalyst so far, and close to the Department of Energy's goal for fuel-cell catalysts: 130 A/cm3. It also matches the performance of typical platinum catalysts. The only problem at the moment is its durability because after only 100 hours of testing the reaction rate dropped to half. Another significant source of losses is the resistance of the membrane to proton flow, which is minimized by making it as thin as possible, on the order of 50 µm.
The PEMFC is a prime candidate for vehicle and other mobile applications of all sizes down to mobile phones, because of its compactness. However, the water management is crucial to performance: too much water will flood the membrane, too little will dry it; in both cases, power output will drop. Water management is a very difficult subject in PEM systems, primarily because water in the membrane is attracted toward the cathode of the cell through polarization. A wide variety of solutions for managing the water exist including integration of electroosmotic pump
Electroosmotic pump
An electroosmotic pump , or EO pump, is used for generating flow or pressure by use of an electric field. One application of this is removing liquid flooding water from channels and gas diffusion layers and direct hydration of the proton exchange membrane in the membrane electrode assembly of the...
s. Furthermore, the platinum catalyst on the membrane is easily poisoned
Catalyst poisoning
Catalyst poisoning refers to the effect that a catalyst can be 'poisoned' if it reacts with another compound that bonds chemically to its active surface sites. This effectively reduces the usefulness of the catalyst...
by carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
(no more than one part per million is usually acceptable) and the membrane is sensitive to things like metal ions, which can be introduced by corrosion of metallic bipolar
Bipolar
-Medicine:* Bipolar cell* Bipolar cell of the retina* Bipolar disorder** Bipolar I disorder** Bipolar II disorder** Bipolar NOS* Bipolar spectrum-Astronomy:* Bipolar nebula, a two-lobed, axially symmetric nebula...
plates, metallic components in the fuel cell system or from contaminants in the fuel/oxidant.
PEM systems that use reformed methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
were proposed, as in Daimler Chrysler Necar 5; reforming methanol, i.e. making it react to obtain hydrogen, is however a very complicated process, that requires also purification from the carbon monoxide the reaction produces. A platinum-ruthenium catalyst is necessary as some carbon monoxide will unavoidably reach the membrane. The level should not exceed 10 parts per million. Furthermore, the start-up times of such a reformer reactor are of about half an hour. Alternatively, methanol, and some other biofuels can be fed to a PEM fuel cell directly without being reformed, thus making a direct methanol fuel cell (DMFC). These devices operate with limited success.
The most commonly used membrane is Nafion
Nafion
Nafion is a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Walther Grot of DuPont. It is the first of a class of synthetic polymers with ionic properties which are called ionomers...
by DuPont
DuPont
E. I. du Pont de Nemours and Company , commonly referred to as DuPont, is an American chemical company that was founded in July 1802 as a gunpowder mill by Eleuthère Irénée du Pont. DuPont was the world's third largest chemical company based on market capitalization and ninth based on revenue in 2009...
, which relies on liquid water humidification of the membrane to transport protons. This implies that it is not feasible to use temperatures above 80 to 90 °C, since the membrane would dry. Other, more recent membrane types, based on Polybenzimidazole (PBI)
Polybenzimidazole fiber
Polybenzimidazole fiber is a synthetic fiber with a very high melting point that also does not readily ignite, because of its exceptional thermal and chemical stability. The U.S...
OR phosphoric acid
Phosphoric-acid fuel cell
Phosphoric acid fuel cells are a type of fuel cell that uses liquid phosphoric acid as an electrolyte. They were the first fuel cells to be commercialized . Developed in the mid-1960s and field-tested since the 1970s, they have improved significantly in stability, performance, and cost...
, can reach up to 220 °C without using any water management: higher temperature allow for better efficiencies, power densities, ease of cooling (because of larger allowable temperature differences), reduced sensitivity to carbon monoxide poisoning and better controllability (because of absence of water management issues in the membrane); however, these recent types are not as common.
Efficiencies of PEMs are in the range of 40–60% higher heating value of hydrogen (HHV).
Catalyst research
Much of the current research on catalysts for PEM fuel cells can be classified as having one of two main objectives:- to obtain higher catalytic activity than the standard carbon-supported platinum particle catalysts used in current PEM fuel cells or
- to reduce the poisoning of PEM fuel cell catalysts by impurity gases. Examples of these two approaches are given in the following sections.
Increasing catalytic activity
As mentioned above, platinum is by far the most effective element used for PEM fuel cell catalysts, and nearly all current PEM fuel cells use platinum particles on porous carbon supports to catalyze both hydrogen oxidation and oxygen reduction. However, due to their high cost, current Pt/C catalysts are not feasible for commercialization. The U.S. Department of Energy estimates that platinum-based catalysts will need to use roughly four times less platinum than is used in current PEM fuel cell designs in order to represent a realistic alternative to internal combustion engines. Consequently, one main goal of catalyst design for PEM fuel cells is to increase the catalytic activity of platinum by a factor of four so that only one-fourth as much of the precious metal is necessary to achieve similar performance.One method of increasing the performance of platinum catalysts is to optimize the size and shape of the platinum particles. Decreasing the particles’ size alone increases the total surface area of catalyst available to participate in reactions per volume of platinum used, but recent studies have demonstrated additional ways to make further improvements to catalytic performance. For example, one study reports that high-index facets of platinum nanoparticles (that is Miller index
Miller index
Miller indices form a notation system in crystallography for planes and directions in crystal lattices.In particular, a family of lattice planes is determined by three integers h, k, and ℓ, the Miller indices. They are written , and each index denotes a plane orthogonal to a direction in the...
es with large integers, such as Pt (730)) provide a greater density of reactive sites for oxygen reduction than typical platinum nanoparticles.
A second method of increasing the catalytic activity of platinum is to alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
it with other metals. For example, it was recently shown that the Pt3Ni(111) surface has a higher oxygen reduction activity than pure Pt(111) by a factor of ten. The authors attribute this dramatic performance increase to modifications to the electronic structure of the surface, reducing its tendency to bond to oxygen-containing ionic species present in PEM fuel cells and hence increasing the number of available sites for oxygen adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
and reduction.
Reducing poisoning
The other popular approach to improving catalyst performance is to reduce its sensitivity to impurities in the fuel source, especially carbon monoxide (CO). Presently, pure hydrogen gas is not economical to mass-produce by electrolysisElectrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
or any other means. Instead, hydrogen gas is produced by steam reforming
Steam reforming
Fossil fuel reforming is a method of producing hydrogen or other useful products from fossil fuels such as natural gas. This is achieved in a processing device called a reformer which reacts steam at high temperature with the fossil fuel. The steam methane reformer is widely used in industry to...
light hydrocarbons, a process which produces a mixture of gasses that also contains CO (1–3%), CO2 (19–25%), and N2 (25%). Even tens of parts per million of CO can poison a pure platinum catalyst, so increasing platinum’s resistance to CO is an active area of research.
For example, one study reported that cube-shaped platinum nanoparticles with (100) faces
Miller index
Miller indices form a notation system in crystallography for planes and directions in crystal lattices.In particular, a family of lattice planes is determined by three integers h, k, and ℓ, the Miller indices. They are written , and each index denotes a plane orthogonal to a direction in the...
displayed a fourfold increase in oxygen reduction activity compared to randomly faceted platinum nanoparticles of similar size. The authors concluded that the (111) facets of the randomly shaped nanoparticles bonded more strongly to sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
ions than the (100) facets, reducing the number of catalytic sites open to oxygen molecules. The nanocubes they synthesized, in contrast, had almost exclusively (100) facets, which are known to interact with sulfate more weakly. As a result, a greater fraction of the surface area of those particles was available for the reduction of oxygen, boosting the catalyst’s oxygen reduction activity.
In addition, researchers have been investigating ways of reducing the CO content of hydrogen fuel before it enters a fuel cell as a possible way to avoid poisoning the catalysts. One recent study revealed that ruthenium-platinum core-shell nanoparticles are particularly effective at oxidizing CO to form CO2, a much less harmful fuel contaminant. The mechanism that produces this effect is conceptually similar to that described for Pt3Ni above: the ruthenium core of the particle alters the electronic structure of the platinum surface, rendering it better able to catalyze the oxidation of CO.
History
Before the invention of PEM fuel cells, existing fuel cell types such as solid-oxide fuel cells were only applied in extreme conditions. Such fuel cells also required very expensive materials and could only be used for stationary applications due to their size. These issues were addressed by the PEM fuel cell. The PEM fuel cell was invented in the early 1960s by Willard Thomas Grubb and Leonard Niedrach of General ElectricGeneral Electric
General Electric Company , or GE, is an American multinational conglomerate corporation incorporated in Schenectady, New York and headquartered in Fairfield, Connecticut, United States...
. Initially, sulfonated polystyrene membranes were used for electrolytes, but they were replaced in 1966 by Nafion
Nafion
Nafion is a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Walther Grot of DuPont. It is the first of a class of synthetic polymers with ionic properties which are called ionomers...
ionomer
Ionomer
An ionomer is a polymer that comprises repeat units of both electrically neutral repeating units and a fraction of ionized units...
, which proved to be superior in performance and durability to sulfonated polystyrene.
PEM fuel cells were used in the NASA
NASA
The National Aeronautics and Space Administration is the agency of the United States government that is responsible for the nation's civilian space program and for aeronautics and aerospace research...
Gemini
Project Gemini
Project Gemini was the second human spaceflight program of NASA, the civilian space agency of the United States government. Project Gemini was conducted between projects Mercury and Apollo, with ten manned flights occurring in 1965 and 1966....
series of spacecraft, but they were replaced by Alkaline fuel cell
Alkaline fuel cell
The alkaline fuel cell , also known as the Bacon fuel cell after its British inventor, is one of the most developed fuel cell technologies. NASA has used alkaline fuel cells since the mid-1960s, in Apollo-series missions and on the Space Shuttle. AFCs consume hydrogen and pure oxygen producing...
s in the Apollo
Project Apollo
The Apollo program was the spaceflight effort carried out by the United States' National Aeronautics and Space Administration , that landed the first humans on Earth's Moon. Conceived during the Presidency of Dwight D. Eisenhower, Apollo began in earnest after President John F...
program and in the Space shuttle
Space Shuttle
The Space Shuttle was a manned orbital rocket and spacecraft system operated by NASA on 135 missions from 1981 to 2011. The system combined rocket launch, orbital spacecraft, and re-entry spaceplane with modular add-ons...
.
Parallel with Pratt and Whitney Aircraft, General Electric developed the first proton exchange membrane fuel cells (PEMFCs) for the Gemini space missions in the early 1960s. The first mission to utilize PEMFCs was Gemini V
Gemini 5
Gemini 5 was a 1965 manned spaceflight in NASA's Gemini program. It was the third manned Gemini flight, the 11th manned American flight and the 19th spaceflight of all time...
. However, the Apollo space missions and subsequent Apollo-Soyuz
Apollo-Soyuz Test Project
-Backup crew:-Crew notes:Jack Swigert had originally been assigned as the command module pilot for the ASTP prime crew, but prior to the official announcement he was removed as punishment for his involvement in the Apollo 15 postage stamp scandal.-Soyuz crew:...
, Skylab
Skylab
Skylab was a space station launched and operated by NASA, the space agency of the United States. Skylab orbited the Earth from 1973 to 1979, and included a workshop, a solar observatory, and other systems. It was launched unmanned by a modified Saturn V rocket, with a mass of...
and Space Shuttle missions utilized fuel cells based on Bacon's design, developed by Pratt and Whitney Aircraft.
Extremely expensive materials were used and the fuel cells required very pure hydrogen and oxygen. Early fuel cells tended to require inconveniently high operating temperatures that were a problem in many applications. However, fuel cells were seen to be desirable due to the large amounts of fuel
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
available (hydrogen and oxygen).
Despite their success in space programs, fuel cell systems were limited to space missions and other special applications, where high cost could be tolerated. It was not until the late 1980s and early 1990s that fuel cells became a real option for wider application base. Several pivotal innovations, such as low platinum catalyst loading and thin film electrodes, drove the cost of fuel cells down, making development of PEMFC systems more realistic. However, there is significant debate as to whether hydrogen fuel cells will be a realistic technology for use in automobile
Automobile
An automobile, autocar, motor car or car is a wheeled motor vehicle used for transporting passengers, which also carries its own engine or motor...
s or other vehicle
Vehicle
A vehicle is a device that is designed or used to transport people or cargo. Most often vehicles are manufactured, such as bicycles, cars, motorcycles, trains, ships, boats, and aircraft....
s. (See hydrogen economy
Hydrogen economy
The hydrogen economy is a proposed system of delivering energy using hydrogen. The term hydrogen economy was coined by John Bockris during a talk he gave in 1970 at General Motors Technical Center....
.)
See also
- Power-to-weight ratioPower-to-weight ratioPower-to-weight ratio is a calculation commonly applied to engines and mobile power sources to enable the comparison of one unit or design to another. Power-to-weight ratio is a measurement of actual performance of any engine or power sources...
- Dynamic hydrogen electrodeDynamic hydrogen electrodeA dynamic hydrogen electrode is a reference electrode, more specific a subtype of the standard hydrogen electrodes for electrochemical processes by simulating a reversible hydrogen electrode with an approximately 20 to 40 mV more negative potential....
- Gas diffusion electrodeGas diffusion electrodeGas diffusion electrodes are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase...
- Hydrogen sulfide sensorHydrogen sulfide sensorA hydrogen sulfide sensor or H2S sensor is a gas sensor for the measurement of hydrogen sulfide.-Principle:The H2S sensor is a metal oxide semiconductor sensor which operates by a reversible change in resistance caused by adsorption and desorption of hydrogen sulfide in a film with hydrogen...
- Reversible hydrogen electrodeReversible hydrogen electrodeA reversible hydrogen electrode is a reference electrode, more specific a subtype of the standard hydrogen electrodes for electrochemical processes and differs from the standard hydrogen electrode by the fact that the measured potential does not change with the pH so that they can be directly used...
- Glossary of fuel cell termsGlossary of fuel cell termsThe Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few. –...
- Timeline of hydrogen technologiesTimeline of hydrogen technologiesTimeline of hydrogen technologies — A timeline of the history of hydrogen technology.-1600s:* 1625 - First description of hydrogen by Johann Baptista van Helmont...

