
Polyphenyl ether
Encyclopedia
Phenyl ether polymers are a class of polymer
s that contain a phenoxy
and/or a thiophenoxy
group as the repeating group in ether
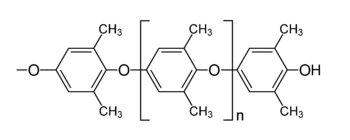
linkages. Commercial phenyl ether polymers belong to two chemical classes: polyphenyl ethers (PPEs) and Polyphenylene oxides (PPOs). The phenoxy groups in the former class of polymers do not contain any substituents whereas those in the latter class contain 2 to 4 alkyl groups on the phenyl ring. The structure of an oxygen-containing PPE is provided in Figure 1 and that of a 2, 6-xylenol derived PPO is shown in Figure 2. Either class can have the oxygen atoms attached at various positions around the rings.

: reaction of an alkali-metal phenate with a halogenated benzene catalyzed by copper.
 PPEs of up to 6 phenyl rings, both oxy and thio ethers, are commercially available. See Table 1. They are characterized by indicating the substitution pattern of each ring, followed by the number of phenyl rings and the number of ether linkages. Thus, the structure in Figure 1 with n equal to 1 is identified as pmp5P4E, indicating para, meta, para substitution of the three middle rings, a total of 5 rings, and 4 ether linkages. Meta substitution of the aryl rings in these materials is most common and often desired. Longer chain analogues with up to 10 benzene rings are also known.
PPEs of up to 6 phenyl rings, both oxy and thio ethers, are commercially available. See Table 1. They are characterized by indicating the substitution pattern of each ring, followed by the number of phenyl rings and the number of ether linkages. Thus, the structure in Figure 1 with n equal to 1 is identified as pmp5P4E, indicating para, meta, para substitution of the three middle rings, a total of 5 rings, and 4 ether linkages. Meta substitution of the aryl rings in these materials is most common and often desired. Longer chain analogues with up to 10 benzene rings are also known.
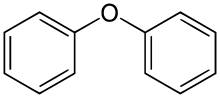
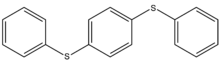
The simplest member of the phenyl ether family is diphenyl ether (DPE), also called diphenyl oxide, the structure of which is provided in Figure 4. Low molecular weight polyphenyl ethers and thioethers are used in a variety of applications and include high vacuum devices, optics, electronics, and in high-temperature and radiation-resistant fluids and greases. Figure 5 show the structure of the sulfur analogue of 3-R polyphenyl ether shown in Figure 3.
The important attributes of PPEs include their thermal and oxidative stability and stability in the presence of ionizing radiation. PPEs have the disadvantage of having somewhat high pour points. For example, PPEs that contain two and three benzene rings are actually solids at room temperatures. The melting points of the ordinarily solid PPEs are lowered if they contain more m-phenylene rings, alkyl groups, or are mixtures of isomers. PPEs that contain only o- and p-substituted rings have the highest melting points.
Oxidation stability of un-substituted PPEs is quite good, partly because they lack easily oxidizable carbon-hydrogen bonds. Thermal decomposition temperature, as measured by the isoteniscope procedure, is between 440 and 465°C.
PPEs have extremely high radiation resistance. Of all classes of synthetic lubricants, with the possible exception of perfluoropolyethers, polyphenyl ethers are the most radiation resistant. Excellent radiation stability of PPEs can be ascribed to the limited number of ionizable carbon-carbon and carbon-hydrogen bonds. In one study, the performance of PPE under the influence of 1011 ergs/gram of radiation at 99°C (210°F) was compared with synthetic ester, synthetic hydrocarbon, and silicone fluids. PPE showed a viscosity increase of only 35%, while all other fluids showed a viscosity increase of 1700% and gelled. Further tests have shown PPEs to be resistant to gamma and associated neutron radiation dosages of 1010erg/g at temperatures up to 600°F.
fluids; high vacuum fluids; and in formulating jet engine/turbine lubricants, high-temperature hydraulic lubricants and greases, and heat transfer fluids. In addition, because of excellent optical properties these fluids have found use in optical devices.
s are devices that remove gases from an enclosed space to greatly reduce pressure, thereby creating a vacuum. While vacuum pumps are of many kinds, oil diffusion pump
s in combination with a fore pump are amongst those most popular. This is because diffusion pumps have the ability to create both high and ultra-high vacuum. Diffusion pumps use a high boiling liquid of low vapor pressure to create a high-speed jet that strikes the gaseous molecules in the system to be evacuated and direct them into space that is being evacuated by the fore pump. Since the diffusion pumps have no moving parts, they are durable and reliable. Major disadvantages of diffusion pumps are the liquid’s tendency to back stream into the vacuum chamber and cause deposit formation by coating the hot surfaces and decomposing.
A good diffusion fluid must therefore reflect low vapor pressure, high flash point, high thermal and oxidative stability and chemical resistance. If the diffusion pump is operating in the proximity of ionizing radiation source, good radiation stability is also desired. Data presented in Table 3 demonstrates polyphenyl ether to be superior to other fluids that are commonly used in diffusion pumps. It is important to note that PPEs help achieve the highest vacuum of 4 x 10-10 torr at 25°C. Such high vacuums are necessary in equipment such as electron microscopes, mass spectrometers and that used for various surface physics studies. Vacuum pumps are also used in the production of electric lamps, vacuum tubes, and cathode ray tubes (CRTs), semiconductor processing, and vacuum engineering.
Polyphenyl ether lubricants have a 30-year history of commercial service for connectors with precious and base metal contacts in telecom, automotive, aerospace, instrumentation and general-purpose applications. In addition to maintaining the current flow and providing long-term lubrication, PPEs offer protection to connectors against aggressive acidic and oxidative environments. By providing a protective surface film, polyphenyl ethers not only protect connectors against corrosion but also against vibration-related wear and abrasion that leads to fretting wear. The devices that benefit from the specialized properties of PPEs include cell phones, printers and a variety of other electronic appliances. The protection lasts for decades or for the life of the equipment.
As noted earlier, PPEs were developed for use in jet engines that involved high speed-related frictional temperatures of as high as 320°C. While the use of PPEs in lubricating jet engines has somewhat subsided due to their higher cost, they are still used in some aerospace applications. PPEs are also used as base fluids for radiation-resistant greases used in nuclear power plant mechanisms. PPEs and their derivatives have also found use as vapor phase lubricants in gas turbines and custom bearings, and wherever extreme environmental conditions exist. Vapor phase lubrication is achieved by heating the liquid lubricant above its boiling point. The resultant vapors are then transported to the hot bearing surface. If the temperatures of the bearing surface are below the lubricant’s boiling point, the vapors condense to provide liquid lubrication. Polyphenyl ether technology can also provide superior fire safety and fatigue life, depending on the specific bearing design. In this application, PPEs have the advantage of providing lubrication both as a liquid at low temperatures and as a vapor at temperatures above 600°F (316°C). Due to the low volatility and excellent high-temperature thermo-oxidative stability, PPEs have also found use as a lubricant for chains used in and around kilns, metal fabrication plants, and glass molding and manufacturing equipment. In these high temperature applications, PPEs do not form any sludge and hard deposits. The low soft carbon residue that is left behind is removed easily by wiping. PPEs low volatility, low flammability, and good thermodynamic properties make them ideally suited for use as heat transfer fluids and in heat sink applications as well.
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s that contain a phenoxy
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
and/or a thiophenoxy
Thiophenol
Thiophenol is an organosulfur compound with the formula C6H6S, and sometimes abbreviated as PhSH. This foul-smelling colourless liquid is the simplest aromatic thiol. The chemical structures of thiophenols are analogous to phenols except the oxygen atom in the hydroxyl group bonded to the...
group as the repeating group in ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
linkages. Commercial phenyl ether polymers belong to two chemical classes: polyphenyl ethers (PPEs) and Polyphenylene oxides (PPOs). The phenoxy groups in the former class of polymers do not contain any substituents whereas those in the latter class contain 2 to 4 alkyl groups on the phenyl ring. The structure of an oxygen-containing PPE is provided in Figure 1 and that of a 2, 6-xylenol derived PPO is shown in Figure 2. Either class can have the oxygen atoms attached at various positions around the rings.


Structure and synthesis of PPEs
The proper name for a phenyl ether polymer is poly(phenyl ether) or polyphenyl polyether, but the name polyphenyl ether is widely accepted. Polyphenyl ethers (PPEs) are obtained by repeated application of the Ullmann Ether SynthesisUllmann condensation
The Ullmann condensation or Ullmann ether synthesis is a variation of the Ullmann reaction, in which a phenol is coupled to an aryl halide to create a diaryl ether in the presence of a copper compound, named after Fritz Ullmann...
: reaction of an alkali-metal phenate with a halogenated benzene catalyzed by copper.

The simplest member of the phenyl ether family is diphenyl ether (DPE), also called diphenyl oxide, the structure of which is provided in Figure 4. Low molecular weight polyphenyl ethers and thioethers are used in a variety of applications and include high vacuum devices, optics, electronics, and in high-temperature and radiation-resistant fluids and greases. Figure 5 show the structure of the sulfur analogue of 3-R polyphenyl ether shown in Figure 3.


| Common and Trade Name | Chemical Name |
|---|---|
| Six-ring Polyphenyl Ether (6P5E); Trade name: OS-138 | Bis[m-(m-phenoxyphenoxy) phenyl] Ether |
| Five-ring Polyphenyl Ether (5P4E); Trade name: OS-124 | m-Bis(m-phenoxyphenoxy)benzene |
| Four-ring Polyphenyl Ether (4P3E); Trade name: MCS-210 | Bis (m-phenoxyphenyl) ether |
| Three- and Four-ring Oxy- and Thioethers ; Trade name: MCS-293 | Thiobis[phenoxybenzene] and Bis (phenylmercapto)benzene |
| Three-ring Polyphenyl Ether (3P2E); Trade name: MCS-2167 | m-Diphenoxybenzene |
| Two-ring Diphenyl Ether (2P1E) | Diphenyl Ether, Diphenyl Oxide, Phenoxybenzene |
Physical properties of PPEs
Typical physical properties of polyphenyl ethers are provided in Table 2. Physical properties of a particular PPE depend upon the number of aromatic rings, their substitution pattern, and whether it is an ether or a thioether. In the case of products of mixed structures, properties are hard to predict from only the structural features; hence, they must be determined via measurement.The important attributes of PPEs include their thermal and oxidative stability and stability in the presence of ionizing radiation. PPEs have the disadvantage of having somewhat high pour points. For example, PPEs that contain two and three benzene rings are actually solids at room temperatures. The melting points of the ordinarily solid PPEs are lowered if they contain more m-phenylene rings, alkyl groups, or are mixtures of isomers. PPEs that contain only o- and p-substituted rings have the highest melting points.
| Polyphenyl Ether | Appearance | Pour Point °F (°C) |
Thermal Stability °F (°C) |
Viscosity (cSt) at 100°F (38°C) |
Viscosity (cSt) at 210°F (99°C) |
|---|---|---|---|---|---|
| 6-Ring 6P5E | Clear Liquid | 50 (10) | 836 (447) | 2000 | 25 |
| 5-Ring 5P4E | Clear Liquid | 40 (4.5) | 847 (453) | 360 | 13 |
| 4-Ring 4P3E | Clear Liquid | 10 (-12) | 825 (441) | 70 | 6 |
| 3- and 4-Ring Oxythio | Hazy Liquid | -20 (-29) | 693 (367) | 25 | 4 |
| 3-Ring 3P2E | Solid | - | 800 (427) | 12 | 3 |
| 2-Ring 2P1E | Solid | - | >600 (316) | 2.4 | 1.6 |
Thermo-oxidative stability
PPEs have excellent high temperature properties and good oxidation stability. With respect to volatilities, p-derivatives have the lowest volatilities and o-derivatives have the highest volatilities. The opposite is true for flash points and fire points. Spontaneous ignition temperatures of polyphenyl ethers lie between 550 and 595°C, alkyl substitution reduces this value by ~50°C. PPEs are compatible with most metals and elastomers that are commonly used in high-temperature applications. They typically swell common seal materials .Oxidation stability of un-substituted PPEs is quite good, partly because they lack easily oxidizable carbon-hydrogen bonds. Thermal decomposition temperature, as measured by the isoteniscope procedure, is between 440 and 465°C.
Radiation stability
Ionizing radiation affects all organic compounds, causing a change in their properties because radiation attacks covalent bonds that are most prevalent in organic compounds. One result of ionization is that the organic molecules disproportionate to form smaller hydrocarbon molecules as well as larger hydrocarbons molecules. This is reflected by increased evaporation loss, lowering of the flash and fire points and increased viscosity. Other chemical reactions caused by radiation include oxidation and isomerization. The former leads to increased acidity, corrosivity, and coke formation and the latter to a change in viscosity and volatility.PPEs have extremely high radiation resistance. Of all classes of synthetic lubricants, with the possible exception of perfluoropolyethers, polyphenyl ethers are the most radiation resistant. Excellent radiation stability of PPEs can be ascribed to the limited number of ionizable carbon-carbon and carbon-hydrogen bonds. In one study, the performance of PPE under the influence of 1011 ergs/gram of radiation at 99°C (210°F) was compared with synthetic ester, synthetic hydrocarbon, and silicone fluids. PPE showed a viscosity increase of only 35%, while all other fluids showed a viscosity increase of 1700% and gelled. Further tests have shown PPEs to be resistant to gamma and associated neutron radiation dosages of 1010erg/g at temperatures up to 600°F.
Surface tension
PPEs have high surface tension; hence these fluids have a lower tendency to wet metal surfaces. The surface tension of the commercially available 5R4E is 49.9 dynes/cm, one of the highest in pure organic liquids . This property is useful in applications where migration of the lubricant into the surrounding environment must be avoided.Applications of PPEs
While originally PPEs were developed for use in extreme environments that were experienced in aerospace applications, they are now used in other applications requiring low volatility and excellent thermo-oxidative and ionizing radiation stability. Such applications include use as diffusion pumpDiffusion pump
Diffusion pumps use a high speed jet of vapor to direct gas molecules in the pump throat down into the bottom of the pump and out the exhaust. Presented in 1915 by Wolfgang Gaede and using mercury vapor, they were the first type of high vacuum pumps operating in the regime of free molecular flow,...
fluids; high vacuum fluids; and in formulating jet engine/turbine lubricants, high-temperature hydraulic lubricants and greases, and heat transfer fluids. In addition, because of excellent optical properties these fluids have found use in optical devices.
Ultra-high-vacuum fluids
Vacuum pumpVacuum pump
A vacuum pump is a device that removes gas molecules from a sealed volume in order to leave behind a partial vacuum. The first vacuum pump was invented in 1650 by Otto von Guericke.- Types :Pumps can be broadly categorized according to three techniques:...
s are devices that remove gases from an enclosed space to greatly reduce pressure, thereby creating a vacuum. While vacuum pumps are of many kinds, oil diffusion pump
Diffusion pump
Diffusion pumps use a high speed jet of vapor to direct gas molecules in the pump throat down into the bottom of the pump and out the exhaust. Presented in 1915 by Wolfgang Gaede and using mercury vapor, they were the first type of high vacuum pumps operating in the regime of free molecular flow,...
s in combination with a fore pump are amongst those most popular. This is because diffusion pumps have the ability to create both high and ultra-high vacuum. Diffusion pumps use a high boiling liquid of low vapor pressure to create a high-speed jet that strikes the gaseous molecules in the system to be evacuated and direct them into space that is being evacuated by the fore pump. Since the diffusion pumps have no moving parts, they are durable and reliable. Major disadvantages of diffusion pumps are the liquid’s tendency to back stream into the vacuum chamber and cause deposit formation by coating the hot surfaces and decomposing.
A good diffusion fluid must therefore reflect low vapor pressure, high flash point, high thermal and oxidative stability and chemical resistance. If the diffusion pump is operating in the proximity of ionizing radiation source, good radiation stability is also desired. Data presented in Table 3 demonstrates polyphenyl ether to be superior to other fluids that are commonly used in diffusion pumps. It is important to note that PPEs help achieve the highest vacuum of 4 x 10-10 torr at 25°C. Such high vacuums are necessary in equipment such as electron microscopes, mass spectrometers and that used for various surface physics studies. Vacuum pumps are also used in the production of electric lamps, vacuum tubes, and cathode ray tubes (CRTs), semiconductor processing, and vacuum engineering.
| Fluid Property | Polyphenyl Ether SANTOVAC 5 |
Silicone Dow Corning |
Hydrocarbon Oil Apiezon |
|---|---|---|---|
| Vapor Pressure, Torr at 25°C | 4x10−10 | 2x10−8 | 5x10−6 |
| Molecular Weight | 446 | 484 | 420 |
| Density at 25°C | 1.20 | 1.07 | 0.87 |
| Flash Point, °C | 288 | 221 | 243 |
| Boiling Point at 1.3 mbar, °C | 295 | 223 | 220 |
| Viscosity (cSt) at 25°C | 1000 | 40 | 135 |
| Viscosity (cSt) at 100°C | 12.0 | 4.3 | 7.0 |
| Surface Tension, Dynes/cm | 49.9 | 30.5 | 30.5 |
| Refractive Index at 25°C, 589 nm | 1.67 | 1.56 | 1.48 |
| Thermal Stability | Excellent | Good | Poor |
| Oxidation Resistance | Excellent | Excellent | Poor-Fair |
| Chemical Resistance | Excellent | Good | Poor |
| Radiation Resistance | Excellent | Good | Fair |
Electronic connector lubricants
5R4E PPE has a surface tension of 49.9 dynes/cm, which is amongst the highest in pure organic liquids. Because of this, this PPE and the other PPEs do not effectively wet metal surfaces. This property is useful when migration of a lubricant from one part of the equipment to another part must be avoided, such as in certain electronic devices. A thin film of polyphenyl ether on a surface is not a thin contiguous film as one would envision, but rather comprises tiny droplets. This PPE property tends to keep the film stationary, or at least to cause it to remain in the area where the lubrication is needed, rather than migrating away by spreading and forming a new surface. As a result, contamination of other components and equipment, which do not require a lubricant, is avoided. The high surface tension of PPEs, therefore, makes them useful in lubricating electronic contacts.Polyphenyl ether lubricants have a 30-year history of commercial service for connectors with precious and base metal contacts in telecom, automotive, aerospace, instrumentation and general-purpose applications. In addition to maintaining the current flow and providing long-term lubrication, PPEs offer protection to connectors against aggressive acidic and oxidative environments. By providing a protective surface film, polyphenyl ethers not only protect connectors against corrosion but also against vibration-related wear and abrasion that leads to fretting wear. The devices that benefit from the specialized properties of PPEs include cell phones, printers and a variety of other electronic appliances. The protection lasts for decades or for the life of the equipment.
Optics
Polyphenyl ethers (PPEs) possess good optical clarity, a high refractive index, and other beneficial optical properties. Because of these, PPEs have the ability to meet the rigorous performance demands of signal processing in advanced photonics systems. Optical clarity of PPEs resembles that of the other optical polymers, that is, they have refractive indices of between 1.5 and 1.7 and provide good propagation of light between approximately 400 nm and 1700 nm. Close refractive index (RI) matching between materials is important for proper propagation of light through them. Because of the ease of RI matching, PPEs are used in many optical devices as optical fluids. Extreme resistance to ionizing radiation gives PPEs an added advantage in the manufacture of solar cells and solid-state UV/blue emitters and telecommunication equipment made from high-index glasses and semiconductors.High-temperature and radiation-resistant lubricants
PPEs, being of excellent thermo-oxidative stability and radiation resistance, have found extensive use in high temperature applications that also require radiation resistance. In addition, PPEs demonstrate better wear control and load-carrying ability than mineral oils, especially when used in bearings.As noted earlier, PPEs were developed for use in jet engines that involved high speed-related frictional temperatures of as high as 320°C. While the use of PPEs in lubricating jet engines has somewhat subsided due to their higher cost, they are still used in some aerospace applications. PPEs are also used as base fluids for radiation-resistant greases used in nuclear power plant mechanisms. PPEs and their derivatives have also found use as vapor phase lubricants in gas turbines and custom bearings, and wherever extreme environmental conditions exist. Vapor phase lubrication is achieved by heating the liquid lubricant above its boiling point. The resultant vapors are then transported to the hot bearing surface. If the temperatures of the bearing surface are below the lubricant’s boiling point, the vapors condense to provide liquid lubrication. Polyphenyl ether technology can also provide superior fire safety and fatigue life, depending on the specific bearing design. In this application, PPEs have the advantage of providing lubrication both as a liquid at low temperatures and as a vapor at temperatures above 600°F (316°C). Due to the low volatility and excellent high-temperature thermo-oxidative stability, PPEs have also found use as a lubricant for chains used in and around kilns, metal fabrication plants, and glass molding and manufacturing equipment. In these high temperature applications, PPEs do not form any sludge and hard deposits. The low soft carbon residue that is left behind is removed easily by wiping. PPEs low volatility, low flammability, and good thermodynamic properties make them ideally suited for use as heat transfer fluids and in heat sink applications as well.

