
Polymer separators
Encyclopedia
A polymer separator is a permeable membrane
placed between the anode
and cathode
of a battery. The main function of a separator is to keep the positive and negative electrodes, the cathode and anode respectively, apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers which are needed to complete the circuit during the passage of current in an electrochemical cell
.
and electrode materials, while also being mechanically strong enough to withstand the high tension of battery construction. They are important to batteries because their structure and properties considerably affect the battery performance, including the batteries energy and power densities, cycle life, and safety.
Dr. Yoshino et al. of the Asahi Kasei Corporation first developed a prototype of secondary lithium-ion batteries (LIBs) in 1983.
These prototype rechargeable cells included two electrodes; the cathode and anode. Initially, lithium cobalt oxide
was used as the cathode and polyacetylene
as the anode. Later in 1985, it was found that using lithium cobalt oxide as the cathode and graphite
as the anode produced an excellent secondary battery based on both enhanced battery stability and the frontier electron theory of Dr. Kenichi Fukui This enabled the development of portable equipment, such as cell phones and laptops. However, before lithium ion batteries could be mass produced for widespread use, safety concerns needed to be addressed such as overheating and over potential. One key to ensuring safety has been the use of a separator between the cathode and anode. This prevents physical contact between the two electrodes while still enabling ionic transport. Furthermore, Dr. Yoshino developed a microporous polyethylene
membrane separator with a “fuse” function. In the case of abnormal heat generation within the battery cell, the separator provides a shutdown mechanism in which the micropores of the separator close by melting and the ionic flow instantly terminates. In 2004, a novel electroactive polymer separator with the function of overcharge protection was first proposed by Dr. Denton et al. This kind of separator can switch reversibly between insulating and conducting states in response of the changes in charge potential based on the intrinsic properties of the conducting polymer. Therefore, one can see how polymer separator’s function and purpose has changed over time. Now, a separators primary function is to provide a protection mechanism for a battery while also effectively transporting ionic charge carriers between the two electrodes as well as preventing the electric contact between them.
, nylon
, polyesters, glass
), polymer films (polyethylene
, polypropylene
, poly (tetrafluoroethylene), poly (vinyl chloride), and naturally occurring substances (rubber
, asbestos
, wood
). There are also ion exchange membranes which are fabricated from polymeric materials that have pores with diameters of less than 20 Å. These are not typically used in batteries because their pore size is too small. The methods for manufacturing the microporous membranes and ion exchange membranes can be divided into two processes: dry process and wet processes.
and other additives and then heating to produce a homogenous solution, then forcing the heated solution through a sheet die into a gel-like film, and then finally extracting the paraffin oil and other additives with a volatile solvent to form the microporous structure.
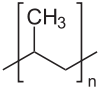 There are specific types of polymers which are ideal for the different types of synthesis. Most of polymers currently used in battery separators are polyolefin
There are specific types of polymers which are ideal for the different types of synthesis. Most of polymers currently used in battery separators are polyolefin
based materials with semi-crystalline structure. Among them, polyethylene
, polypropylene
, and their blends such as polyethylene-polypropylene are widely used. Recently, graft polymers have been studied in an attempt to improve battery performance, including micro-porous poly(methyl methacrylate)-grafted and siloxane
grafted polyethylene separators, which show favorable surface morphology and electrochemical properties as compared to conventional polyethylene separators. In addition, poly(vinylidene fluoride) (PVDF) nanofiber webs can be synthesized as a separator to improve both ion conductivity and dimensional stability Another type of polymer separator, polytriphenylamine (PTPAn)-modified separator, is an electroactive separator with reversible overcharge protection.
. These include but are not limited to: semi-crystalline polyolefins, polyoxymethylene
, and isotactic poly (4-methyl-1-pentene). One can also use blends of two immiscible polymers, in which at least one polymer has a crystalline structure, such as polyethylene-polypropylene, polystyrene-polypropylene, and poly (ethylene terephthalate) - polypropylene blends.
.
occurs. Even though the separator must be able to shut down at particular temperatures, it must be able to retain its mechanical properties.
electric vehicle
s. These types of vehicles need to have lithium-ion batteries that contain high energy and power density. In other words, if one were to accelerate a full electric vehicle the secondary cell needs to output a large amount of energy as quickly as possible. DupontTM has introduced a novel nano-fiber based polymeric battery separator that boosts the performance and safety of lithium-ion batteries. These types of separators, named EnergainTM, are potential candidates for use in full electric vehicles in the near future. The EnergainTM separators are synthesized into a web using a proprietary spinning process that creates continuous filaments. These filaments can range in diameter from 200 – 1,000 nanometers. The separators exhibit stability and low shrinkage in high temperatures and are easily saturated in common organic electrolytes. This results in more efficient operation, longer battery life, and improved safety. Batteries containing this type of separator can be quickly recharged, deliver improved performance, and reduce the number of cells needed by up to thirty-three percent for hybrid electric vehicles. Overall, this type of polymer separator can increase power up to thirty percent. Also, the battery life can also be increased by twenty percent. This is due to their stability at high temperatures and the overall morphology of the separator. With more battery power, drivers can travel farther on a single charge and accelerate more quickly and safely.
As seen previously, polymer separators are of great importance, especially in the area of lithium-ion batteries. Jun Young Kim at Massachusetts Institute of Technology used plasma technology to modify a polyethylene membrane to create a high performance separator for practical applications in rechargeable lithium ion polymer batteries. Plasma treatment methods have been developed to modify polymer surfaces for enhanced adhesion, wettability, and printability. These are usually performed by modifying the surfaces on only several molecular levels. This allows the surface functionalization of polymers without sacrificing the bulk properties. The surface of the polyethylene membrane was modified with acrylonitrile via plasma coating technique. The lithium-ion polymer cell that contained the plasma induced acrylonitrile coated polyethylene (PiAn-PE) membrane was analyzed using various spectroscopic techniques. The surface characterization demonstrated that the enhanced adhesion of PiAN-PE membrane resulted from the increased polar component of surface energy. The presence of PiAN induced onto the surface of PE membrane via plasma modification process plays a crucial role in improving the wettability and electrolyte retention, the interfacial adhesion between the electrodes and the separator, and the cycle performance of the resulting lithium-ion polymer cell assembly. This plasma-modified PE membrane holds a great potential to be a promising polymer membrane as a high-performance and cost-effective separator for lithium-ion polymer batteries.
These polymer separators are also used in other secondary cells.
Another example of a secondary cell is the sealed rechargeable nickel/metal hydride battery. This offers significant improvement over conventional rechargeable batteries in terms of performance and environmental friendliness. The Ni/MH, like the lithium-ion battery, has the ability to display high energy and power density. These batteries have long cycle lives making them a leading technology as a battery source for electric vehicles. However, the greatest problem of Ni/MH cells are their inherent high corrosion rate in aqueous solutions. As a contribution to alkaline Ni/MH secondary battery technology, there has been a strong demand to replace the conventional aqueous electrolyte by a solid or gel polymer electrolyte/separator.
In Ni/MH cells, the most commonly used separators are porous insulator films of polyolefin, nylon, or cellophane. Another way to modify these porous insulator films is the process of radiation grafting. Acrylic compounds can be radiation-grafted onto these separators to make their properties more desirable i.e. more wettable and permeable to the electrolyte. Zhijiang Cai and co-workers developed a solid polymer membrane gel separator. This was a polymerization product of one or more monomers selected from the group of water-soluble ethylenically unsaturated amides and acid. The polymer-based gel also includes a water swellable polymer, which acts as a reinforcing element. In addition, ionic species are added to the solution and remain embedded in the polymer gel after polymerization. Recently, more and more Ni/MH batteries of bipolar design are being developed because they offer some advantages for applications as high power storage systems for electric vehicles. It was found that this solid polymer membrane gel separator could be very useful for such applications in bipolar design. In other words, this design can help in avoiding short-circuits occurring in liquid-electrolyte systems.
Inorganic polymer separators have also been of interest as use in lithium-ion batteries. Inorganic particulate film/poly(methyl methacrylate) (PMMA)/inorganic particulate film trilayer separators are prepared by means of simple dip-coating
of inorganic particle layers on to both sides of PMMA thin films. This inorganic trilayer membrane is believed to be an inexpensive, novel separator for application in lithium-ion batteries due to the increased dimensional and thermal stability.
Artificial membrane
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of twentieth century. A...
placed between the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
and cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
of a battery. The main function of a separator is to keep the positive and negative electrodes, the cathode and anode respectively, apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers which are needed to complete the circuit during the passage of current in an electrochemical cell
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
.
Background
Polymer separators are critical components in liquid electrolyte batteries. The separator is placed between the positive and negative electrode in order to prevent physical contact of the electrodes while enabling ionic transport. A separator generally consists of a polymeric membrane forming a microporous layer. It must be chemically and electrochemically stable towards the electrolyteElectrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
and electrode materials, while also being mechanically strong enough to withstand the high tension of battery construction. They are important to batteries because their structure and properties considerably affect the battery performance, including the batteries energy and power densities, cycle life, and safety.
History
Unlike many forms of technology, polymer separators were not developed specifically for batteries. They were instead a result of spin-offs of existing technologies, which is why most polymer separators are not optimized for many of the systems they are used in. Even though this may seem unfavorable, most polymer separators can be mass produced at a comparatively low cost, because they are based on existing forms of technologies.Dr. Yoshino et al. of the Asahi Kasei Corporation first developed a prototype of secondary lithium-ion batteries (LIBs) in 1983.
These prototype rechargeable cells included two electrodes; the cathode and anode. Initially, lithium cobalt oxide
Lithium cobalt oxide
Lithium cobalt oxide is a chemical compound commonly used in the positive electrodes of lithium-ion batteries. The structure of LiCoO2 is known theoretically and has been confirmed with techniques like x-ray diffraction, electron microscopy, neutron powder diffraction, and EXAFS: it consists of...
was used as the cathode and polyacetylene
Polyacetylene
Polyacetylene is an organic polymer with the repeat unit n. The high electrical conductivity discovered for these polymers beginning in the 1960's accelerated interest in the use of organic compounds in microelectronics...
as the anode. Later in 1985, it was found that using lithium cobalt oxide as the cathode and graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
as the anode produced an excellent secondary battery based on both enhanced battery stability and the frontier electron theory of Dr. Kenichi Fukui This enabled the development of portable equipment, such as cell phones and laptops. However, before lithium ion batteries could be mass produced for widespread use, safety concerns needed to be addressed such as overheating and over potential. One key to ensuring safety has been the use of a separator between the cathode and anode. This prevents physical contact between the two electrodes while still enabling ionic transport. Furthermore, Dr. Yoshino developed a microporous polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
membrane separator with a “fuse” function. In the case of abnormal heat generation within the battery cell, the separator provides a shutdown mechanism in which the micropores of the separator close by melting and the ionic flow instantly terminates. In 2004, a novel electroactive polymer separator with the function of overcharge protection was first proposed by Dr. Denton et al. This kind of separator can switch reversibly between insulating and conducting states in response of the changes in charge potential based on the intrinsic properties of the conducting polymer. Therefore, one can see how polymer separator’s function and purpose has changed over time. Now, a separators primary function is to provide a protection mechanism for a battery while also effectively transporting ionic charge carriers between the two electrodes as well as preventing the electric contact between them.
Synthesis
Polymer separators generally fall in the category of microporous polymer membranes. Microporous polymer membranes are usually fabricated from a variety of inorganic, organic, and naturally occurring materials. The pore size in these types of polymer separators is typically larger than 50-100 Å. Materials such as nonwoven fibers (cottonCotton
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective capsule, around the seeds of cotton plants of the genus Gossypium. The fiber is almost pure cellulose. The botanical purpose of cotton fiber is to aid in seed dispersal....
, nylon
Nylon
Nylon is a generic designation for a family of synthetic polymers known generically as polyamides, first produced on February 28, 1935, by Wallace Carothers at DuPont's research facility at the DuPont Experimental Station...
, polyesters, glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
), polymer films (polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
, polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
, poly (tetrafluoroethylene), poly (vinyl chloride), and naturally occurring substances (rubber
Rubber
Natural rubber, also called India rubber or caoutchouc, is an elastomer that was originally derived from latex, a milky colloid produced by some plants. The plants would be ‘tapped’, that is, an incision made into the bark of the tree and the sticky, milk colored latex sap collected and refined...
, asbestos
Asbestos
Asbestos is a set of six naturally occurring silicate minerals used commercially for their desirable physical properties. They all have in common their eponymous, asbestiform habit: long, thin fibrous crystals...
, wood
Wood
Wood is a hard, fibrous tissue found in many trees. It has been used for hundreds of thousands of years for both fuel and as a construction material. It is an organic material, a natural composite of cellulose fibers embedded in a matrix of lignin which resists compression...
). There are also ion exchange membranes which are fabricated from polymeric materials that have pores with diameters of less than 20 Å. These are not typically used in batteries because their pore size is too small. The methods for manufacturing the microporous membranes and ion exchange membranes can be divided into two processes: dry process and wet processes.
Dry Process
The dry process consists of three steps: extruding, annealing, and stretching. The extruding step is generally carried out at a temperature higher than the melting point of the polymer resin. This is because the polymer resins are melted in order to shape them into a uniaxially orientated tubular film, called a precursor film. The structure and orientation of the precursor film produced depends on the processing conditions and the characteristics of the polymer resin used. In the next step, the annealing process, the precursor polymer is annealed at a temperature slightly lower than the melting point of the polymer. The purpose of this step is to improve the crystalline structure in order to enable the formation of micropores in the final step, stretching. In the final step, stretching, the annealed film is deformed along the machine direction by a process consisting of a cold stretch, a hot stretch, and a relaxation. The cold stretch is used to create the pore structure by stretching the film at a lower temperature with a faster strain rate, and the hot stretch is to increase the size of the pores by further stretching the film at a higher temperature with a slower strain rate. The purpose of the relaxation step is to reduce internal stress within the film. The porosity of the final film depends on the morphology of the precursor film, annealing conditions, and the stretching ratios and conditions.Wet Process
Similar to the dry process the wet process consists of three steps: the mixing of the polymer resins, paraffin oil, antioxidantAntioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
and other additives and then heating to produce a homogenous solution, then forcing the heated solution through a sheet die into a gel-like film, and then finally extracting the paraffin oil and other additives with a volatile solvent to form the microporous structure.
Different types of polymers used in batteries

Polyolefin
A polyolefin is a polymer produced from a simple olefin as a monomer. For example, polyethylene is the polyolefin produced by polymerizing the olefin ethylene. An equivalent term is polyalkene; this is a more modern term, although polyolefin is still used in the petrochemical industry...
based materials with semi-crystalline structure. Among them, polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
, polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
, and their blends such as polyethylene-polypropylene are widely used. Recently, graft polymers have been studied in an attempt to improve battery performance, including micro-porous poly(methyl methacrylate)-grafted and siloxane
Siloxane
A siloxane is any chemical compound composed of units of the form R2SiO, where R is a hydrogen atom or a hydrocarbon group. They belong to the wider class of organosilicon compounds....
grafted polyethylene separators, which show favorable surface morphology and electrochemical properties as compared to conventional polyethylene separators. In addition, poly(vinylidene fluoride) (PVDF) nanofiber webs can be synthesized as a separator to improve both ion conductivity and dimensional stability Another type of polymer separator, polytriphenylamine (PTPAn)-modified separator, is an electroactive separator with reversible overcharge protection.
Ideal Polymers for Dry Processes
The dry process is only suitable for polymers with high crystallinityCrystallinity
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a big influence on hardness, density, transparency and diffusion. In a gas, the relative positions of the atoms or...
. These include but are not limited to: semi-crystalline polyolefins, polyoxymethylene
Polyoxymethylene
Polyoxymethylene , also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts that require high stiffness, low friction and excellent dimensional stability....
, and isotactic poly (4-methyl-1-pentene). One can also use blends of two immiscible polymers, in which at least one polymer has a crystalline structure, such as polyethylene-polypropylene, polystyrene-polypropylene, and poly (ethylene terephthalate) - polypropylene blends.
Ideal Polymers for Wet Processes
The wet process is suitable for both crystalline and amorphous polymers. The separators synthesized by wet processes often use ultrahigh-molecular-weight polyethylene. The use of these polymers enables the batteries to have favorable mechanical properties while also preventing the battery from functioning when it becomes too hot.Wet process vs. Dry process
Membranes synthesized by dry processes are more suitable for a high power density battery because they have an open and uniform pore structure, while those made by wet processes are more suited for a long cycle life battery because of their tortuous and interconnected porous structure. This helps to suppress the growth of Li crystals on the graphite anode during fast charging or low temperature charging.Placement of Polymer Separators in Batteries
The separator is placed between the anode and the cathode. The pores of the separator are filled with the electrolyte. The electrode and separator combination is then wound into tight rolls which are then fitted into rigid cylindrical or prismatic (rectangular) metal cans.Chemical Stability
The separator material must be chemically stable against the electrolyte and electrode materials, especially under the strongly reductive and oxidative environments when the battery is fully charged. The separator should not degrade and lose mechanical strength. One can determine the chemical stability of a polymer separator by calendar life testing.Thickness of Separator
A battery separator must be relatively thin in order to facilitate the high energy and power densities of the battery. However, if the separator is too thin, it can decrease the mechanical strength and safety of the battery. Additionally, a separator should have uniform thickness in order to support the long like cycle of a battery. In current technologies, 25.4μm is generally accepted as the standard width. The thickness of a polymer separator can be measured using the T411 om-83 method developed under the auspices of the Technical Association of the Pulp and Paper Industry.Porosity of Separator
The separator must have the correct amount of porosity in order to hold a sufficient amount of liquid electrolyte in order to enable the movement of ions between the electrodes. The porosity cannot be too high because this hinders the ability of the pores to close, which is a vital component of the separators ability to shut down a battery. The porosity can be measured using liquid or gas absorption methods according to the American Society for Testing and Materials (ASTM) D-2873. Typically, a Li-ion battery separator will have a porosity of 40%.Pore Size
Pore size is also very important to the functioning of the separator. The pore size must be smaller than the particle size of the electrode components, including the electrode active materials and the conducting additives. Ideally the pores should be uniformly distributed while also having a tortuous structure. This ensures a uniform current distribution throughout the separator while suppressing the growth of Li on the anode. The distribution and structure of pores can be analyzed using a Capillary Flow Porometer or a Scanning Electron MicroscopeScanning electron microscope
A scanning electron microscope is a type of electron microscope that images a sample by scanning it with a high-energy beam of electrons in a raster scan pattern...
.
Permeability
The separator should not limit the electrical performance of the battery. Usually the presence of a polymer separator will increase the resistance of the electrolyte by a factor of four to five. The ratio of the resistance of the separator filled with electrolyte divided by the resistance of the electrolyte alone is called the MacMullin number. Air permeability can be used indirectly to estimate the MacMullin number. Air permeability is expressed in terms of the Gurley value, which is defined as the time required for a specific amount of air to pass through a specific area of the separator under a specific pressure. The Gurley value reflects the tortuosity of the pores, when the porosity and thickness of the separators are fixed. A separator with uniform porousness is vital to the long life cycle of a battery. Deviations from uniform permeability will result in uneven current density distribution, which causes the formation of Li crystals on the graphite anode.Mechanical Strength
The separator must be strong enough to withstand the tension of the winding operation during battery assembly. The mechanical strength of the polymer separator is also very important. Mechanical strength is typically defined in terms of the tensile strength in two directions, the machine direction and the transverse direction, and terms of the tear resistance and puncture strength. All of these parameters are defined in terms of Young’s modulus.Wetability
The electrolyte must be able to fill the entire battery assembly therefore, it is important that the separator wet easily when submerged in the electrolyte. Furthermore, the separator should be able to retain the electrolyte permanently, which increases the cycle life of the battery. There is not a generally accepted method used to test wettability, other than placing a droplet of electrolyte onto the separator and observing what happens.Stability
It is important that the separator remain stable over a wide temperature range. It is essential that once the separator is soaked with electrolyte it lays completely flat.Thermal Capabilities
Another major requirement for separators in lithium-ion batteries is the ability to shut down at a temperature slightly lower than that at which thermal runawayThermal runaway
Thermal runaway refers to a situation where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result...
occurs. Even though the separator must be able to shut down at particular temperatures, it must be able to retain its mechanical properties.
Defects
Many Structural defects can form in polymer separators due to temperature changes. These structural defects can result in a thicker separators. Furthermore, there can be intrinsic defects in the polymers themselves, such as polyethylene often begins to deteriorate during the stages of polymerization, transportation, and storage. Additionally, defects such as tears or holes can form during the synthesis of polymer separators. There are also other sources of defects can come from doping the polymer separator. Recently groups have been trying to improve the wetability of the polyer separators by co-dopping the normal polyethylene separator with acrylonitrile. The researchers found that acrylonitrile was more susceptible to be compatible with the electrolyte due to the wettability property.Use in Li-ion Batteries
Polymer separators, similar to battery separators in general, act as a separator of the anode and cathode in the Li-ion battery while also enabling the movement of ions through the cell. Additionally, many of the polymer separators, typically multilayer polymer separators, can act as “shutdown separators”, which are able to shut down the battery if it becomes too hot during the cycling process. These multilayered polymer separators are generally composed of one or more polyethylene layers which serve to shut down the battery and at least one polypropylene layers which acts as a form of mechanical support for the separator.Other types of battery separators
In addition to polymer separators, there are several other types of separators. There are nonwovens, which consist of a manufactured sheet, web, or matt of directionally or randomly oriented fibers. Supported liquid membranes, which consist of a solid and liquid phase contained within a microporous separator. Additionally there are also polymer electrolytes which can form complexes with different types of alkali metal salts, which results in the production of ionic conductors which serve as solid electrolytes. Another type of separator, a solid ion conductor, can serve as both a separator and the electrolyte in a battery.Advancements in Polymer Separators
The topic of polymer separators has been an active area of research due to the application of lithium-ion batteries in full or hybridHybrid electric vehicle
A hybrid electric vehicle is a type of hybrid vehicle and electric vehicle which combines a conventional internal combustion engine propulsion system with an electric propulsion system. The presence of the electric powertrain is intended to achieve either better fuel economy than a conventional...
electric vehicle
Electric vehicle
An electric vehicle , also referred to as an electric drive vehicle, uses one or more electric motors or traction motors for propulsion...
s. These types of vehicles need to have lithium-ion batteries that contain high energy and power density. In other words, if one were to accelerate a full electric vehicle the secondary cell needs to output a large amount of energy as quickly as possible. DupontTM has introduced a novel nano-fiber based polymeric battery separator that boosts the performance and safety of lithium-ion batteries. These types of separators, named EnergainTM, are potential candidates for use in full electric vehicles in the near future. The EnergainTM separators are synthesized into a web using a proprietary spinning process that creates continuous filaments. These filaments can range in diameter from 200 – 1,000 nanometers. The separators exhibit stability and low shrinkage in high temperatures and are easily saturated in common organic electrolytes. This results in more efficient operation, longer battery life, and improved safety. Batteries containing this type of separator can be quickly recharged, deliver improved performance, and reduce the number of cells needed by up to thirty-three percent for hybrid electric vehicles. Overall, this type of polymer separator can increase power up to thirty percent. Also, the battery life can also be increased by twenty percent. This is due to their stability at high temperatures and the overall morphology of the separator. With more battery power, drivers can travel farther on a single charge and accelerate more quickly and safely.
As seen previously, polymer separators are of great importance, especially in the area of lithium-ion batteries. Jun Young Kim at Massachusetts Institute of Technology used plasma technology to modify a polyethylene membrane to create a high performance separator for practical applications in rechargeable lithium ion polymer batteries. Plasma treatment methods have been developed to modify polymer surfaces for enhanced adhesion, wettability, and printability. These are usually performed by modifying the surfaces on only several molecular levels. This allows the surface functionalization of polymers without sacrificing the bulk properties. The surface of the polyethylene membrane was modified with acrylonitrile via plasma coating technique. The lithium-ion polymer cell that contained the plasma induced acrylonitrile coated polyethylene (PiAn-PE) membrane was analyzed using various spectroscopic techniques. The surface characterization demonstrated that the enhanced adhesion of PiAN-PE membrane resulted from the increased polar component of surface energy. The presence of PiAN induced onto the surface of PE membrane via plasma modification process plays a crucial role in improving the wettability and electrolyte retention, the interfacial adhesion between the electrodes and the separator, and the cycle performance of the resulting lithium-ion polymer cell assembly. This plasma-modified PE membrane holds a great potential to be a promising polymer membrane as a high-performance and cost-effective separator for lithium-ion polymer batteries.
These polymer separators are also used in other secondary cells.
Another example of a secondary cell is the sealed rechargeable nickel/metal hydride battery. This offers significant improvement over conventional rechargeable batteries in terms of performance and environmental friendliness. The Ni/MH, like the lithium-ion battery, has the ability to display high energy and power density. These batteries have long cycle lives making them a leading technology as a battery source for electric vehicles. However, the greatest problem of Ni/MH cells are their inherent high corrosion rate in aqueous solutions. As a contribution to alkaline Ni/MH secondary battery technology, there has been a strong demand to replace the conventional aqueous electrolyte by a solid or gel polymer electrolyte/separator.
In Ni/MH cells, the most commonly used separators are porous insulator films of polyolefin, nylon, or cellophane. Another way to modify these porous insulator films is the process of radiation grafting. Acrylic compounds can be radiation-grafted onto these separators to make their properties more desirable i.e. more wettable and permeable to the electrolyte. Zhijiang Cai and co-workers developed a solid polymer membrane gel separator. This was a polymerization product of one or more monomers selected from the group of water-soluble ethylenically unsaturated amides and acid. The polymer-based gel also includes a water swellable polymer, which acts as a reinforcing element. In addition, ionic species are added to the solution and remain embedded in the polymer gel after polymerization. Recently, more and more Ni/MH batteries of bipolar design are being developed because they offer some advantages for applications as high power storage systems for electric vehicles. It was found that this solid polymer membrane gel separator could be very useful for such applications in bipolar design. In other words, this design can help in avoiding short-circuits occurring in liquid-electrolyte systems.
Inorganic polymer separators have also been of interest as use in lithium-ion batteries. Inorganic particulate film/poly(methyl methacrylate) (PMMA)/inorganic particulate film trilayer separators are prepared by means of simple dip-coating
Dip-coating
thumb|A schematic of the continuous dip coating process.Dip coating is a popular way of creating thin films for research purposes. Uniform films can be applied onto flat or cylindrical substrates...
of inorganic particle layers on to both sides of PMMA thin films. This inorganic trilayer membrane is believed to be an inexpensive, novel separator for application in lithium-ion batteries due to the increased dimensional and thermal stability.

