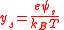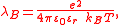Polyelectrolyte adsorption
Encyclopedia
Adsorption
of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups (dubbed polyelectrolytes) bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.
, which models the interaction of charged particles in solution, and mean field theory
, which simplifies systems for analysis.
Using a modified Poisson-Boltzmann equation
and a mean field equation, the concentration profile near a charged surface is solved numerically. The solution of these equations yields a simple relation for the adsorbed amount, Γ, based on electrolyte charge fraction, ρ, and bulk salt concentration,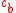 .
.
where is the reduced surface potential:
is the reduced surface potential:
and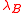 is the Bjerrum length
is the Bjerrum length
:
effect of the solvent and the steric attraction or repulsion facilitated by the chemical species of the solvent and its temperature. Repulsive steric forces are based on entropy and are caused by the reduced configuration entropy of the polymer chains. It is difficult to model precisely the interaction that any particular polyelectrolye solution will exhibit because the steric forces are dependent upon the combination of the chemical makeup of both the polymer and the solvent as well as any ionic species present in the solution.
using the layer-by-layer application method in order to inhibit corrosion. The exact mechanism by which corrosion is restricted is unknown due to the fact that polyelectrolyte multilayers are water-logged and of a gel-like consistency. One theory is that the layers form a barrier impenetrable to small ions that facilitate corrosion of the steel. Additionally, the water molecules within the multilayer film are held in a restricted state by the ionic groups of the polyelectrolytes. This decreases the chemical activity of the water at the surface of the steel.
, which is a matrix of sessile bacteria consisting of around 15% bacterial cells by mass and 85% hydrophobic exopolysaccharide fibers. One way to eliminate this risk is to apply localized treatment to the area in the vicinity of the implant. This can be done by applying a drug-impregnated polyelectrolyte multilayer to the medical device prior to implantation. The goal with this technology is to create a combination of polyelectrolyte multilayers where one multilayer prevents the formation of a biofilm and another releases a small-molecule drug through diffusion. This would be more effective than the current technique of releasing a high dose of drugs into the body and counting on some of it to navigate to the afflicted area. The base layer for an effective coating for an implant is DMLPEI/PAA, or linear N, N-dodecyl,methyl-poly(ethyleneimine) / poly(acrylic acid).
. These forces tend to cause colloidal particles to come out of solution by either flocculation
or coagulation. The Hamaker attractive effect is balanced by one or both of two repulsive effects of colloids in solution. The first is electrostatic stabilization, in which like charges of the particles repel one another. This effect is due to the zeta potential
that exists due to a particle's surface charge in solution. The second is steric stabilization, due to steric effects
. Drawing particles together with adsorbed polymer chains greatly decreases the conformational entropy
of the polymer chains at the surface, which is thermodynamically unfavorable, making flocculation and coagulation more difficult.
The adsorption of polyelectrolytes can be used to stabilize the suspension, such as in the case of dyes and paints. It can also be used to destabilize the suspension by adsorbing oppositely charged chains to the particle surface, neutralizing the zeta-potential, causing flocculation or coagulation of contaminants. This is used heavily in waste-water treatment to force suspensions of contaminants to flocculate, allowing them to be filtered. There are a variety of industrial flocculants that are either cationic or anionic in nature for targeting particular species.
These cores, once created, can be used for things such as drug delivery
or as a microreactor
. For drug delivery, the polyelectrolyte shell would breakdown after a certain amount of time, releasing the drug then and helping the drug travel through the digestive tract, which is one of the biggest barriers for the effectiveness of delivering a drug.
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups (dubbed polyelectrolytes) bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.
Kinetics of Layer Formation
Models for the adsorption behavior of polyelectrolytes in solution to a solid surface are extremely situational. Vastly different behaviors are exhibited based on varying polyelectrolyte character and concentration, ionic strength of the solution, solid surface character, pH, among several others. These complex models are specialized for certain parameters by application in order to create accurate models.Theoretical Kinetics of Polyelectrolyte Adsorption
However, the general character of the process can be reasonably well modeled with a polyelectrolyte in solution, and an oppositely charged surface where no covalent interaction between the surface and chain occurs. This model for the adsorbed amount of polyelectrolyte at a charged surface is derived from DLVO theoryDLVO theory
The DLVO theory is named after Derjaguin and Landau, Verwey and Overbeek.The theory describes the force between charged surfaces interacting through a liquid medium....
, which models the interaction of charged particles in solution, and mean field theory
Mean field theory
Mean field theory is a method to analyse physical systems with multiple bodies. A many-body system with interactions is generally very difficult to solve exactly, except for extremely simple cases . The n-body system is replaced by a 1-body problem with a chosen good external field...
, which simplifies systems for analysis.
Using a modified Poisson-Boltzmann equation
Poisson-Boltzmann equation
The Poisson–Boltzmann equation is a differential equation that describes electrostatic interactions between molecules in ionic solutions. It is the mathematical base for the Gouy–Chapman double layer theory; first proposed by Gouy in 1910 and complemented by Chapman in 1913...
and a mean field equation, the concentration profile near a charged surface is solved numerically. The solution of these equations yields a simple relation for the adsorbed amount, Γ, based on electrolyte charge fraction, ρ, and bulk salt concentration,
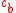 .
.where
 is the reduced surface potential:
is the reduced surface potential:and
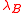 is the Bjerrum length
is the Bjerrum lengthBjerrum length
The Bjerrum length is the separation at which the electrostatic interaction between twoelementary charges is comparable in magnitude to the thermal energy scale,...
:
Layer-by-Layer Adsorption
Because charge plays a key role in polyelectrolyte adsorption, initial rates of adsorption of polyectrolytes to charged surfaces are often rapid, limited only by the rate of mass-transport (diffusion) to the surface. This high rate then quickly drops off as charge accumulation at the surface occurs, and attractive forces are no longer drawing more polyelectrolyte chains to the surface. This drop in adsorption rates can be countered by exploiting the tendency for charge overcompensation to occur. In the case of a negatively charged solid surface, cationic polyelectrolate chains are adsorbed to the oppositely charged surface. Their large size and high charge densities tend to overcompensate the original negative surface charge, resulting in a net positive charge due to the cationic polyelectrolytes. This solid surface, with its cationic polyelectrolyte film, and consequent positive surface charge, can then be exposed to an anionic polyelectrolyte solution, where the process begins again, creating another film with an oppositely charged surface. This process can then be repeated to create several bilayers on the solid surface.Effects of Contents and Quality of the Solution
The effectiveness of polyelectrolyte adsorption is greatly affected by the contents of the solution and by the quality of the solvent in which the polyelectrolytes are dissolved. The primary mechanism by which the solvent affects the adsorption characteristics of the surface/polymer interface is due to the dielectricDielectric
A dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric is placed in an electric field, electric charges do not flow through the material, as in a conductor, but only slightly shift from their average equilibrium positions causing dielectric...
effect of the solvent and the steric attraction or repulsion facilitated by the chemical species of the solvent and its temperature. Repulsive steric forces are based on entropy and are caused by the reduced configuration entropy of the polymer chains. It is difficult to model precisely the interaction that any particular polyelectrolye solution will exhibit because the steric forces are dependent upon the combination of the chemical makeup of both the polymer and the solvent as well as any ionic species present in the solution.
Solvent Choice
The interactions between a polyelectrolyte and the solvent it is place in have a large effect on the conformation of the polymer both in solution and upon deposition onto the substrate. Due to their unique nature, polyelectrolytes have many options for solvents that traditional polymers such as polyethylene, styrene, and others, would not be soluble in. An excellent example of this is water. While water is a high polarity solvent, it will still dissolve many polyelectrolytes. The conformation of a polyelectrolyte in solution is determined by a balancing act of the (usually unfavorable) interactions between the solvent and the polymer and the electrostatic repulsions felt by the individual repeat units of the polymer. It has been suggested that in order to optimize its energy, a polyelectrolyte chain will form into an elongated cylindrical globule. Some models go further and postulate that the most efficient configuration is a series of cylindrical globules linking much larger diameter spherical globules in a "necklace" configuration.Good Solvent
In a good solvent, the electrostatic forces between the repeat units of the polymer and the solvent are favorable. While not entirely intuitive, this causes the polymer to assume a more tightly packed conformation. This is due to the screening the solvent molecules perform between the charged repeat units of the polyelectolyte, decreasing the electostatic repulsion the polymer chain experiences. Since the polymer backbone does not repel itself as strongly as it would in a poor solvent, the polymer chain acts more similarly to an uncharged polymer, assuming a compact conformation.Poor Solvent
In a poor solvent, the solvent molecules interact poorly or unfavorably with the charged portions of the polyelectrolyte. The inability of the solvent to effectively screen the charges between repeat units causes the polymer to assume a looser conformation due to electrostatic repulsions of the repeat units. These interactions will allow for the polymer to be more uniformly deposited onto the substrate.Salt Concentration
When an ionic compound is dissolved in the solvent, the ions act to screen the charges attached to the polyelectrolyte chains. The ionic concentration of the solution will determine the layer formation characteristics of the polyelectrolyte as well as the conformation the polymer assumes in solution.High Salt
High salt concentrations cause conditions similar to the interactions experienced by a polymer in a favorable solvent. Polyelectrolytes, while charged, are still mainly non-polar carbon backboned polymers. While the charges on the polymer backbone exert an electrostatic force that drives the polymer into a more open and loose conformation, if the surrounding solution has a high concentration of salt then the charge repulsion will be screened. Once this charge is screened the polyelectrolyte will react as any other non-polar polymer would react in a high ionic strength solution and begin to minimize interactions with the solvent. This leads to a much more clumped and dense deposition of the polymer onto the surface.Low Salt
In a low ionic strength solution, the charges present on the repeat units of the polymer are the dominant force controlling conformation. Since there is very little charge present to screen the repulsive interactions between the repeat units, the polymer assumes a very spread out, loose conformation. This conformation allows for more uniform layering on the substrate, which is helpful in preventing surface defects and non-uniform surface properties.Industrial Uses of Polyelectrolyte Layers
Polyelectrolytes can be applied to multiple types of surfaces due to the variety of ionic polymers available. They can be applied to solid surfaces in multilayer form to fulfill a variety of design objectives, they can be used to surround solid particles to enhance the stability of a colloidal system, and they can even be assembled to form an independent structure that can be used to ferry drugs throughout the human body.| Polyelectrolyte | Full Name | Application |
|---|---|---|
| polyDADMAC | polydiallyldimethylammonium chloride | heavy waste water flocculant |
| PAH-Naf / PAH-PAA | poly(allylamine)-Nafion / poly(acrylic acid) | mechanically responsive variable hydrophobicity film |
| DMLPEI/PAA | linear N, N-dodecyl,methyl-poly(ethyleneimine) / poly(acrylic acid) | microbicidal coating |
| PEI | poly(ethyleneimine) | anchoring layer for biosensor Biosensor A biosensor is an analytical device for the detection of an analyte that combines a biological component with a physicochemical detector component.It consists of 3 parts:* the sensitive biological element A biosensor is an analytical device for the detection of an analyte that combines a biological... electrode Electrode An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit... |
| PSS | poly(styrene sulfonate) | bilayer component for biosensor coating |
| PAH | poly(allylamine hydrochloride) | bilayer component for biosensor coating |
| PAH-PAA | poly(allylamine / poly(acrylic acid) | pH-induced controlled delivery of methylene blue |
| PAA/PEO-b-PCL | poly(acrylic acid) / polyethylene oxide - block - polycaprolactone | Triclosan drug delivery through degradation release. |
Polymer Coatings
Polyelectrolyte multilayers are a promising area of research in the polymer coating industry because they can be applied in a spray-on fashion at low cost in a water-based solvent. It has been determined that although the polymers are held to the adsorbent only by electrostatic forces, the multilayer coatings adhere aggressively under liquid shear. The disadvantage to this coating technology is that the layers have the consistency of a gel and thus are weak against abrasion.Stainless Steel Corrosion Resistance
Polyelectrolytes have been used by scientists to coat stainless steelStainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
using the layer-by-layer application method in order to inhibit corrosion. The exact mechanism by which corrosion is restricted is unknown due to the fact that polyelectrolyte multilayers are water-logged and of a gel-like consistency. One theory is that the layers form a barrier impenetrable to small ions that facilitate corrosion of the steel. Additionally, the water molecules within the multilayer film are held in a restricted state by the ionic groups of the polyelectrolytes. This decreases the chemical activity of the water at the surface of the steel.
Implant Enhancement
Many biomedical devices that come into contact with bodily fluids are susceptible to adverse foreign body response, or rejection, and thus failure of the device. The main mechanism of infection is the formation of a biofilmBiofilm
A biofilm is an aggregate of microorganisms in which cells adhere to each other on a surface. These adherent cells are frequently embedded within a self-produced matrix of extracellular polymeric substance...
, which is a matrix of sessile bacteria consisting of around 15% bacterial cells by mass and 85% hydrophobic exopolysaccharide fibers. One way to eliminate this risk is to apply localized treatment to the area in the vicinity of the implant. This can be done by applying a drug-impregnated polyelectrolyte multilayer to the medical device prior to implantation. The goal with this technology is to create a combination of polyelectrolyte multilayers where one multilayer prevents the formation of a biofilm and another releases a small-molecule drug through diffusion. This would be more effective than the current technique of releasing a high dose of drugs into the body and counting on some of it to navigate to the afflicted area. The base layer for an effective coating for an implant is DMLPEI/PAA, or linear N, N-dodecyl,methyl-poly(ethyleneimine) / poly(acrylic acid).
Colloid Stability
Another of the major applications of polyelectrolyte adsorption is the stabilization (or destabilization) of solid colloidal suspensions, or sols. Particles in solution tend to have attractive forces similar to van der Waals forces, modeled by Hamaker theoryHamaker theory
After the explanation of van der Waals forces by Fritz London several scientists soon realised that his definition could be extended from the interaction of two molecules with induced dipoles to macro-scale objects by summing all of the forces between the molecules in each of the bodies involved. ...
. These forces tend to cause colloidal particles to come out of solution by either flocculation
Flocculation
Flocculation, in the field of chemistry, is a process wherein colloids come out of suspension in the form of floc or flakes by the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended in a liquid and not actually...
or coagulation. The Hamaker attractive effect is balanced by one or both of two repulsive effects of colloids in solution. The first is electrostatic stabilization, in which like charges of the particles repel one another. This effect is due to the zeta potential
Zeta potential
Zeta potential is a scientific term for electrokinetic potential in colloidal systems. In the colloidal chemistry literature, it is usually denoted using the Greek letter zeta, hence ζ-potential...
that exists due to a particle's surface charge in solution. The second is steric stabilization, due to steric effects
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
. Drawing particles together with adsorbed polymer chains greatly decreases the conformational entropy
Conformational entropy
Conformational entropy is the entropy associated with the physical arrangement of a polymer chain that assumes a compact or globular state in solution. The concept is most commonly applied to biological macromolecules such as proteins and RNA, but can also be used for polysaccharides and other...
of the polymer chains at the surface, which is thermodynamically unfavorable, making flocculation and coagulation more difficult.
The adsorption of polyelectrolytes can be used to stabilize the suspension, such as in the case of dyes and paints. It can also be used to destabilize the suspension by adsorbing oppositely charged chains to the particle surface, neutralizing the zeta-potential, causing flocculation or coagulation of contaminants. This is used heavily in waste-water treatment to force suspensions of contaminants to flocculate, allowing them to be filtered. There are a variety of industrial flocculants that are either cationic or anionic in nature for targeting particular species.
Encapsulation of Liquid Cores
An application of the additional stability a polyelectrolyte multilayer will grant a colloid is the creation of a solid coating for a liquid core. While polyelectrolyte layers are generally adsorbed onto solid substrates, they may also be adsorbed to liquid substrates such as oil in water emulsions or colloids. This process has tons of potential, but is rife with difficulty. Since colloids are generally stabilized by surfactants, and oftentimes ionic surfactants, the adsorption of the multilayer that is similarly charged to the surfactant causes problems due to the electrostatic repulsions felt by the polyelectrolyte and the surfactants. This can be circumvented by using non-ionic surfactants; however, the solubility of these non-ionic surfactants in water is greatly decreased compared to ionic surfactants.These cores, once created, can be used for things such as drug delivery
Drug delivery
Drug delivery is the method or process of administering a pharmaceutical compound to achieve a therapeutic effect in humans or animals. Drug delivery technologies modify drug release profile, absorption, distribution and elimination for the benefit of improving product efficacy and safety, as well...
or as a microreactor
Microreactor
A microreactor or microstructured reactor or microchannel reactor is a device in which chemical reactions take place in a confinement with typical lateral dimensions below 1 mm;the most typical form of such confinement are microchannels...
. For drug delivery, the polyelectrolyte shell would breakdown after a certain amount of time, releasing the drug then and helping the drug travel through the digestive tract, which is one of the biggest barriers for the effectiveness of delivering a drug.