
Plum pudding model
Encyclopedia
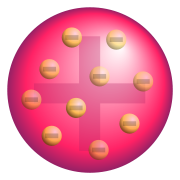
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
by J. J. Thomson
J. J. Thomson
Sir Joseph John "J. J." Thomson, OM, FRS was a British physicist and Nobel laureate. He is credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer...
, who discovered the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
in 1897, was proposed in 1904 before the discovery of the atomic nucleus. In this model, the atom is composed of electrons (which Thomson still called "corpuscles", though G. J. Stoney had proposed that atoms of electricity be called electrons in 1894) surrounded by a soup of positive charge to balance the electrons' negative charges, like negatively-charged "plum
Plum
A plum or gage is a stone fruit tree in the genus Prunus, subgenus Prunus. The subgenus is distinguished from other subgenera in the shoots having a terminal bud and solitary side buds , the flowers in groups of one to five together on short stems, and the fruit having a groove running down one...
s" surrounded by positively-charged "pudding
Pudding
Pudding most often refers to a dessert, but it can also be a savory dish.In the United States, pudding characteristically denotes a sweet milk-based dessert similar in consistency to egg-based custards, though it may also refer to other types such as bread and rice pudding.In the United Kingdom and...
". The electrons (as we know them today) were thought to be positioned throughout the atom, but with many structures possible for positioning multiple electrons, particularly rotating rings of electrons (see below). Instead of a soup, the atom was also sometimes said to have had a "cloud" of positive charge.
With this model, Thomson abandoned his earlier "nebular atom" hypothesis in which the atom was composed of immaterial vorticies. Now, at least part of the atom was to be composed of Thomson's particulate negative corpuscles, although the rest of the positively-charged part of the atom remained somewhat nebulous and ill-defined.
The 1904 Thomson model was disproved by the 1909 gold foil experiment
Geiger-Marsden experiment
The Geiger–Marsden experiment was an experiment to probe the structure of the atom performed by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester...
, which was interpreted by Ernest Rutherford
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
in 1911
to imply a very small nucleus of the atom containing a very high positive charge (in the case of gold, enough to balance about 100 electrons), thus leading to the Rutherford model
Rutherford model
The Rutherford model or planetary model is a model of the atom devised by Ernest Rutherford. Rutherford directed the famous Geiger-Marsden experiment in 1909, which suggested on Rutherford's 1911 analysis that the so-called "plum pudding model" of J. J. Thomson of the atom was incorrect...
of the atom. Although gold has an atomic number of 79, immediately after Rutherford's paper appeared in 1911 Antonius Van den Broek
Antonius Van den Broek
Antonius Johannes van den Broek was a Dutch amateur physicist notable for being the first who realized that the number of an element in the periodic table corresponds to the charge of its atomic nucleus....
made the intuitive suggestion that atomic number is nuclear charge. The matter required experiment to decide. Henry Moseley
Henry Moseley
Henry Gwyn Jeffreys Moseley was an English physicist. Moseley's outstanding contribution to the science of physics was the justification from physical laws of the previous empirical and chemical concept of the atomic number. This stemmed from his development of Moseley's law in X-ray spectra...
's work showed experimentally in 1913 (see Moseley's law
Moseley's law
Moseley's law is an empirical law concerning the characteristic x-rays that are emitted by atoms. The law was discovered and published by the English physicist Henry Moseley in 1913...
) that the effective nuclear charge was very close to the atomic number (Moseley found only one unit difference), and Moseley referenced only the papers of Van den Broek and Rutherford. This work culminated in the solar-system-like (but quantum-limited) Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of the atom in the same year, in which a nucleus containing an atomic number of positive charge is surrounded by an equal number of electrons in orbital shells. Bohr had also inspired Moseley's work.
Thomson's model was compared (though not by Thomson) to a British dessert called plum pudding
Christmas pudding
Christmas pudding is a pudding traditionally served on Christmas Day . It has its origins in medieval England, and is sometimes known as plum pudding or plum duff, though this can also refer to other kinds of boiled pudding involving dried fruit.-Basics:Many households have their own recipe for...
, hence the name. Thomson's paper was published in the March 1904 edition of the Philosophical Magazine
Philosophical Magazine
The Philosophical Magazine is one of the oldest scientific journals published in English. Initiated by Alexander Tilloch in 1798, in 1822 Richard Taylor became joint editor and it has been published continuously by Taylor & Francis ever since; it was the journal of choice for such luminaries as...
, the leading British science journal of the day. In Thomson's view:
... the atoms of the elements consist of a number of negatively electrified corpuscles enclosed in a sphere of uniform positive electrification, ...
In this model, the electrons were free to rotate within the blob or cloud of positive substance. These orbits were stabilized in the model by the fact that when an electron moved farther from the center of the positive cloud, it felt a larger net positive inward force, because there was more material of opposite charge, inside its orbit (see Gauss's law
Gauss's law
In physics, Gauss's law, also known as Gauss's flux theorem, is a law relating the distribution of electric charge to the resulting electric field. Gauss's law states that:...
). In Thomson's model, electrons were free to rotate in rings which were further stabilized by interactions between the electrons, and spectra were to be accounted for by energy differences of different ring orbits. Thomson attempted to make his model account for some of the major spectral lines known for some elements, but was not notably successful at this. Still, Thomson's model (along with a similar Saturnian ring model for atomic electrons, also put forward in 1904 by Nagaoka after James Clerk Maxwell
James Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
's model of Saturn's rings), were earlier harbingers of the later and more successful solar-system-like Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of the atom.

