
Photophosphorylation
Encyclopedia
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
) reactions. All organisms produce ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, which is the universal energy currency of life.
In photophosphorylation, light energy is used to create a high-energy electron donor and a lower-energy electron acceptor. Electrons then move spontaneously from donor to acceptor through an electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
.
Background
ATP is made by an enzymeEnzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
called ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
. Both the structure of this enzyme and its underlying gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
are remarkably similar in all known forms of life.
ATP synthase is powered by a transmembrane electrochemical potential gradient
Potential gradient
A potential gradient is the local space rate of change of the potential with respect to displacement.In electrostatics then, it is the local space rate of change of the electric potential:Units are volts per meter...
, usually in the form of a proton gradient. The function of the electron transport chain is to produce this gradient. In all living organisms, a series of redox reactions is used to produce a transmembrane electrochemical potential gradient, or a so-called proton motive force (pmf).
Redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions are chemical reactions in which electrons are transferred from a donor molecule to an acceptor molecule. The underlying force driving these reactions is the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of the reactants and products. The Gibbs free energy is the energy available (“free”) to do work. Any reaction that decreases the overall Gibbs free energy of a system will proceed spontaneously (given that the system is isobaric and also adiabatic)
The transfer of electrons from a high-energy molecule (the donor) to a lower-energy molecule (the acceptor) can be spatially separated into a series of intermediate redox reactions. This is an electron transport chain.
The fact that a reaction is thermodynamically possible does not mean that it will actually occur. A mixture of hydrogen gas and oxygen gas does not spontaneously ignite. It is necessary either to supply an activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
or to lower the intrinsic activation energy of the system, in order to make most biochemical reactions proceed at a useful rate. Living systems uszlex macromolecular structures to lower the activation energies of biochemical reactions.
It is possible to couple a thermodynamically favorable reaction (a transition from a high-energy state to a lower-energy state) to a thermodynamically unfavorable reaction (such as a separation of charges, or the creation of an osmotic gradient), in such a way that the overall free energy of the system decreases (making it thermodynamically possible), while useful work is done at the same time. Biological macromolecules that catalyze a thermodynamically favorable reaction if and only if a thermodynamically unfavorable reaction occurs simultaneously underlie all known forms of life.
Electron transport chains (most known as ETC) produce energy in the form of a transmembrane electrochemical potential gradient. This energy is used to do useful work. The gradient can be used to transport molecules across membranes. It can be used to do mechanical work, such as rotating bacterial flagella. It can be used to produce ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
and NADPH, high-energy molecules that are necessary for growth.
Cyclic photophosphorylation
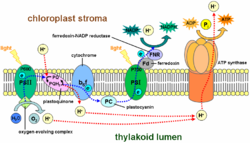
Cylic photophosphorylation occurs on the thylakoid membrane. In cyclic electron flow, the electron begins in a pigment complex called photosystem I, passes from the primary acceptor to ferredoxinFerredoxin
Ferredoxins are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co...
, then to cytochrome b6f (a similar complex to that found in mitochondria), and then to plastocyanin
Plastocyanin
Plastocyanin is an important copper-containing protein involved in electron-transfer. The protein is monomeric, with a molecular weight around 10,500 Daltons, and 99 amino acids in most vascular plants...
before returning to chlorophyll. This transport chain produces a proton-motive force, pumping H+ ions across the membrane; this produces a concentration gradient that can be used to power ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
during chemiosmosis
Chemiosmosis
Chemiosmosis is the movement of ions across a selectively permeable membrane, down their electrochemical gradient. More specifically, it relates to the generation of ATP by the movement of hydrogen ions across a membrane during cellular respiration....
. This pathway is known as cyclic photophosphorylation, and it produces neither O2 nor NADPH. Unlike non-cyclic photophosphorylation, NADP+ does not accept the electrons, but they are sent back to photosystem I. NADPH is NOT produced in cyclic photophosphorylation.
In bacterial photosynthesis, a single photosystem is used, and therefore is involved in cyclic photophosphorylation.
It is favoured in anaerobic conditions and conditions of high irradiance and CO2 compensation point.
Noncyclic photophosphorylation
The other pathway, noncyclic photophosphorylation, is a two-stage process involving two different chlorophyll photosystems. Being a light reaction, Noncyclic photophosphorylation occurs on thylakoidThylakoid
A thylakoid is a membrane-bound compartment inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as...
membranes inside chloroplasts. First, a water molecule is broken down into 2H+ + 1/2 O2 + 2e- by a process called photolysis (or light-splitting). The two electrons from the water molecule are kept in photosystem II, while the 2H+ and 1/2O2 are left out for further use. Then a photon is absorbed by chlorophyll pigments on surrounding the reaction core center of the photosystem. The light excites the electrons of each pigment, causing a chain reaction that eventually transfers energy to the core of photosystem II, exciting the two electrons that are transferred to the primary electron acceptor, pheophytin. The deficit of electrons is replenished by taking electrons from another molecule of water. The electrons transfer from pheophytin to plastoquinone, then to plastocyanin, providing the energy for hydrogen ions (H+) to be pumped into the thylakoid space. This creates a gradient, making H+ ions flow back into the stroma of the chloroplast, providing the energy for the regeneration of ATP.
The photosystem II complex replaced its lost electrons from an external source; however, the two other electrons are not returned to photosystem II as they would in the analogous cyclic pathway. Instead, the still-excited electrons are transferred to a photosystem I complex, which boosts their energy level to a higher level using a second solar photon. The highly excited electrons are transferred to the acceptor molecule, but this time are passed on to an enzyme called Ferredoxin- NADP reductase|NADP+ reductase, for short FNR, which uses them to catalyse the reaction (as shown):
- NADP+ + 2H+ + 2e- → NADPH + H+
This consumes the H+ ions produced by the splitting of water, leading to a net production of 1/2O2, ATP, and NADPH+H+ with the consumption of solar photons and water.
The concentration of NADPH in the chloroplast may help regulate which pathway electrons take through the light reactions. When the chloroplast runs low on ATP for the Calvin cycle, NADPH will accumulate and the plant may shift from noncyclic to cyclic electron flow.

