
Phosphorus sulfide
Encyclopedia
The Phosphorus sulfides comprise a family of inorganic compound
s containing only phosphorus
and sulfur
. These compounds have the formula P4Sx where x is less than or equal to 10. Two are of commercial significance, phosphorus pentasulfide
(P2S5), which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide
(P4S3), used in the production of "strike anywhere matches."
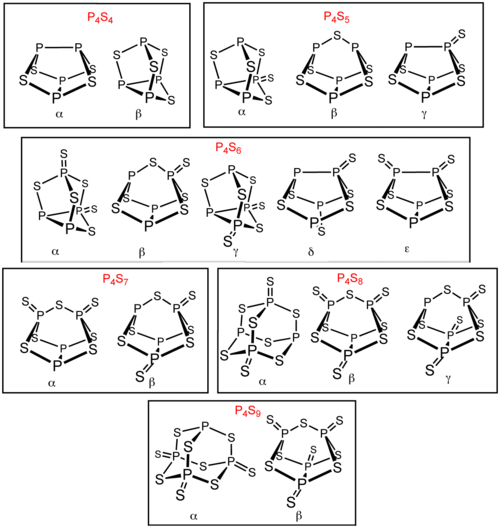
Several other phosphorus sulfides in addition to P4S3 and P4S10. Six of these phosphorus sulfides exist as isomer
s: P4S4, P4S5, P4S6, P4S7, P4S8, and P4S9. These isomers are distinguished by Greek letter prefixes. The prefix is based on the order of the discovery of the isomers, not their structure. All known molecular phosphorus sulfides contain a tetrahedral array of four phosphorus atoms. P4S2 is also known but is unstable above− 30 °C.
. More selective syntheses entail (i) desulfurization, e.g. using triphenylphosphine
and, complementarily, (ii) sulfidation using triphenylarsine
sulfide.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
s containing only phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. These compounds have the formula P4Sx where x is less than or equal to 10. Two are of commercial significance, phosphorus pentasulfide
Phosphorus pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value...
(P2S5), which is made on a kiloton scale for the production of other organosulfur compounds, and phosphorus sesquisulfide
Phosphorus sesquisulfide
Phosphorus sesquisulfide is the inorganic compound with the formula 43. This yellow solid is one of two commercially produced phosphorus sulfides. It is a component of "strike anywhere" matches....
(P4S3), used in the production of "strike anywhere matches."
Several other phosphorus sulfides in addition to P4S3 and P4S10. Six of these phosphorus sulfides exist as isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s: P4S4, P4S5, P4S6, P4S7, P4S8, and P4S9. These isomers are distinguished by Greek letter prefixes. The prefix is based on the order of the discovery of the isomers, not their structure. All known molecular phosphorus sulfides contain a tetrahedral array of four phosphorus atoms. P4S2 is also known but is unstable above

Preparation
The main method for preparing these compounds is thermolysis of mixtures of phosphorus and sulfur. The product distributions can be analyzed by 31P NMR spectroscopyNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
. More selective syntheses entail (i) desulfurization, e.g. using triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
and, complementarily, (ii) sulfidation using triphenylarsine
Triphenylarsine
Triphenylarsine is the chemical compound with the formula As3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis...
sulfide.

