
Phosphoramidite
Encyclopedia
Nucleoside
Nucleosides are glycosylamines consisting of a nucleobase bound to a ribose or deoxyribose sugar via a beta-glycosidic linkage...
s. They are used to synthesize
Oligonucleotide synthesis
Oligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acids with defined chemical structure . The technique is extremely useful in current laboratory practice because it provides a rapid and inexpensive access to custom-made oligonucleotides of the desired...
oligonucleotide
Oligonucleotide
An oligonucleotide is a short nucleic acid polymer, typically with fifty or fewer bases. Although they can be formed by bond cleavage of longer segments, they are now more commonly synthesized, in a sequence-specific manner, from individual nucleoside phosphoramidites...
s, relatively short fragments of nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
and their analogs
Nucleic acid analogues
Nucleic acid analogues are compounds structurally similar to naturally occurring RNA and DNA, used in medicine and in molecular biology research....
. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers. In order to avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. In order to be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog have to possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides
Nucleic acid analogues
Nucleic acid analogues are compounds structurally similar to naturally occurring RNA and DNA, used in medicine and in molecular biology research....
, LNA
Locked nucleic acid
A locked nucleic acid , often referred to as inaccessible RNA, is a modified RNA nucleotide. The ribose moiety of an LNA nucleotide is modified with an extra bridge connecting the 2' oxygen and 4' carbon. The bridge "locks" the ribose in the 3'-endo conformation, which is often found in the A-form...
, morpholino
Morpholino
In molecular biology, a Morpholino is a molecule in a particular structural family that is used to modify gene expression. Morpholino oligomers are an antisense technology used to block access of other molecules to specific sequences within nucleic acid...
, nucleosides modified at the 2'-position (OMe, protected NH2, F), nucleosides containing non-canonical bases (hypoxanthine
Hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-Hydroxypurine. Hypoxanthine is a necessary additive in certain cell,...
and xanthine
Xanthine
Xanthine , is a purine base found in most human body tissues and fluids and in other organisms. A number of stimulants are derived from xanthine, including caffeine and theobromine....
contained in natural nucleosides inosine
Inosine
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring via a β-N9-glycosidic bond....
and xanthosine
Xanthosine
Xanthosine is a nucleoside derived from xanthine and ribose.-See also:* Xanthosine monophosphate* Xanthosine diphosphate* Xanthosine triphosphate...
, respectively, tricyclic bases such as G-clamp, etc.) or bases derivatized with a fluorescent group or a linker arm.
Preparation of nucleoside phosphoramidites
There are three main methods for the preparation of nucleoside phosphoramidites.- The method used most commonly consist in the treatment of a protected nucleoside bearing a single free hydroxy group with phosphorodiamidite under the catalytic action of a weak acid. Although some bisamidites were reported as thermally unstable compounds, 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite, the amidite used for the preparation of commercial nucleoside phosphoramidites, is relatively stable. It can be synthesized using a two-step, one-pot procedure and purified by vacuumVacuumIn everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
distillationDistillationDistillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
. An excellent review outlines the use of the latter reagent in preparation of nucleosidic and non-nucleosidic phosphoramidites in great detail.
- In the second method, the protected nucleoside is treated with the phosphorochloridite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base).
- In the third method, the protected nucleoside is first treated with chloro N,N,N',N'-tetraisopropyl phosphorodiamidite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base) to form a protected nucleoside diamidite. The latter is treated with an alcohol respective to the desired phosphite protecting group, for instance, 2-cyanoethanol, in the presence of a weak acid.
Nucleoside phosphoramidites are purified by column chromatography
Column chromatography
Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms.The main advantage of column chromatography is the relatively low cost and disposability...
on silica gel
Silica gel
Silica gel is a granular, vitreous, porous form of silica made synthetically from sodium silicate. Despite its name, silica gel is a solid. It is a naturally occurring mineral that is purified and processed into either granular or beaded form...
. To warrant the stability of the phosphoramidite moiety, it is advisable to maintain 3 to 5% concentration of a weak base (triethylamine or pyridine) in the eluent throughout the entire course of the separation. The purity of a phosphoramidite may be assessed by 31P NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
. As the P(III) atom is chiral, in a nucleoside phosphoramidite it displays two peaks at about 149 ppm corresponding to the two diastereomers of the compound. The potentially present H-phosphonate impurities display peaks at 8 and 10 ppm.
Chemical properties of phosphoramidite moiety
Nucleoside phosphoramidites are relatively stable compounds with a prolonged shelf-life when stored as powders under anhydrous conditions in the absence of air at temperatures below 4oC. The amidites well withstand mild basic conditions. In contrast, in the presence of even mild acids, phosphoramidites perish almost instantaneously. The phosphoramidites are relatively stable to hydrolysis under neutral conditions. For instance, half-lifeHalf-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 2-cyanoethyl 5'-O-(4,4'-dimethoxytrityl)thymidine
Thymidine
Thymidine is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine in double-stranded DNA...
-3'-O-(N,N-diisopropylamino)phosphite in 95% aqueous acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
at 25oC is 200 h.
- The most important feature of phosphoramidites is their ability to undergo the phosphoramidite coupling reaction that is, to react with nucleophilic groups in the presence of an acidic azoleAzoleAn azole is a class of five-membered nitrogen heterocyclic ring compounds containing at least one other non-carbon atom of either nitrogen, sulfur, or oxygen. The parent compounds are aromatic and have two double bonds; there are successively reduced analogs with fewer...
catalyst, 1H-tetrazoleTetrazoleTetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen and one carbon atom . The simplest is tetrazole itself, CN4H2. They are unknown in nature...
, 2-ethylthiotetrazole, 2-benzylthiotetrazole, 4,5-dicyanoimidazoleImidazoleImidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
, or a number of similar compounds. The reaction proceeds extremely rapidly. This very feature makes nucleoside phosphoramidites useful intermediates in oligonucleotide synthesisOligonucleotide synthesisOligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acids with defined chemical structure . The technique is extremely useful in current laboratory practice because it provides a rapid and inexpensive access to custom-made oligonucleotides of the desired...
. Stereochemically, the phosphoramidite coupling leads to the epimerisation (forming of diastereomers) at the P(III) chiral center.
When water is served as a nucleophile, the product is an H-phosphonate diester as shown in Scheme above. Due to the presence of residual water in solvents and reagents, the formation of the latter compound is the most common complication in the preparative use of phosphoramidites, particularly in oligonucleotide synthesis.
- Phosphoramidites are readily oxidized with weak oxidating reagents, for instance, with aqueous iodine in the presence of weak bases or with hydrogen peroxideHydrogen peroxideHydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
to form the respective phosphoramidates.
Similarly, phosphoramidites react with other chalcogen
Chalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
s. When brought in contact with a solution of sulfur or a number of compounds collectively referred to as sulfurizing agents, phosphoramidites quantitatively form phosphorothioamidates. The reaction with selenium or selenium derivatives produces phosphoroselenoamidates. In all reactions of this type, the configuration at the phosphorus atom is retained.
- Nucleoside phosphoramidites undergo Michaelis-Arbuzov reactionMichaelis-Arbuzov reactionThe Michaelis–Arbuzov reaction is the chemical reaction of a trialkyl phosphite and an alkyl halide to form a phosphonate....
to form the respective phosphonamidates. One example describes the preparation of phosphonamidates in the presence of acrylonitrile. Reportedly,, at room temperature the reaction is stereoselective with the retention of configuration at the phosphorus center. In contrast, when carried out ar 55oC, the reaction leads to racemizedRacemizationIn chemistry, racemization refers to the converting of an enantiomerically pure mixture into a mixture where more than one of the enantiomers are present...
products.
- Similarly to phosphines and tertiary phosphites, phosphoramidites readily undergo Staudinger reactionStaudinger reactionThe Staudinger reaction or Staudinger reduction is a chemical reaction in which the combination of an azide with a phosphine or phosphite produces an iminophosphorane intermediate. Combined with the hydrolysis of the aza-ylide to produce a phosphine oxide and an amine, this reaction is a mild...
.
(RO)2P-N(R1)2 + R2-N3 + H2O ---- (RO)2P(=O)-N(R1)2 + R2-NH2 + N2;
Protecting strategy
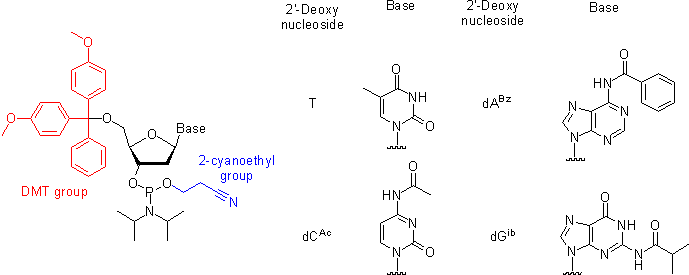
The naturally occurring nucleotides (nucleoside-3'- or 5'-phosphates) and their phosphodiester analogs are insufficiently reactive to afford an expedite synthetic preparation of oligonucleotides in high yields. The selectivity and the rate of the formation of internucleosidic linkages are dramatically improved by using 3'-O-(N,N-diisopropyl phosphoramidite) derivatives of nucleosides (nucleoside phosphoramidites) that serve as building blocks in phosphite triester methodology. To prevent undesired side reactions, all other functional groups present in nucleosides have to be rendered unreactive (protected) by attaching protecting groups. Upon the completion of the oligonucleotide chain assembly, all the protecting groups are removed to yield the desired oligonucleotides. Below, the protecting groups currently used in commercially available and most common nucleoside phosphoramidite building blocks are briefly reviewed:- The 5'-hydroxyl group is protected by an acid-labile DMT (4,4'-dimethoxytrityl) group.
- ThymineThymineThymine is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. As the name suggests, thymine may be derived by methylation of uracil at...
and uracilUracilUracil is one of the four nucleobases in the nucleic acid of RNA that are represented by the letters A, G, C and U. The others are adenine, cytosine, and guanine. In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine.Uracil is a common and...
, nucleic bases of thymidineThymidineThymidine is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine in double-stranded DNA...
and uridineUridineUridine is a molecule that is formed when uracil is attached to a ribose ring via a β-N1-glycosidic bond.If uracil is attached to a deoxyribose ring, it is known as a deoxyuridine....
, respectively, do not have exocyclic amino groups and hence do not require any protection. In contrast, nucleic bases adenineAdenineAdenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
, cytosineCytosineCytosine is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine . It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached . The nucleoside of cytosine is cytidine...
, and guanineGuanineGuanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine . In DNA, guanine is paired with cytosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with...
bear the exocyclic amino groups, which are reactive with the activated phosphoramidites under the conditions of the coupling reaction. Although, at the expense of additional steps in the synthetic cycle, the oligonucleotide chain assembly may be carried out using phosphoramidites with unprotected amino groups, most often these are kept permanently protected over the entire length of the oligonucleotide chain assembly. The protection of the exocyclic amino groups have to be orthogonal to that of the 5'-hydroxy group because the latter is removed at the end of each synthetic cycle. The simplest to implement and hence the most widely accepted is the strategy where the exocyclic amino groups bear a base-labile protection. Most often, two protection schemes are used.
- In the first, the standard and more robust scheme (Figure), Bz (benzoyl) protection is used for A, dA, C, dC, G, and dG are protected with isobutyryl group. More recently, Ac (acetyl) group is often used to protect C and dC as shown in Figure.
- In the second, mild protection scheme, A and dA are protected with isobutyryl or phenoxyacetyl groups (PAC). C and dC bear acetyl protection, and G and dG are protected with 4-isopropylphenoxyacetyl (i-Pr-PAC) or dimethylformamidino (dmf) groups. Mild protecting groups are removed more readily than the standard protecting groups. However, the phosphoramidites bearing these groups are less stable when stored in solution.
- The phosphite group is protected by a base-labile 2-cyanoethyl group. Once a phosphoramidite has been coupled to the solid support-bound oligonucleotide and the phosphite moieties have been converted to the P(V) species, the presence of the phosphate protection is not mandatory for the successful conducting of further coupling reactions.
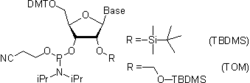
- In RNA synthesis, the 2'-hydroxy group is protected with TBDMS (t-butyldimethylsilyl) group. or with TOM (tri-iso-propylsilyloxymethyl) group, both being removable by treatment with fluoride ion.
- The phosphite moiety also bears a diisopropylamino (iPr2N) group reactive under acidic conditions. Upon activation, the diisopropylamino group leaves to be substituted by the 5'-hydroxy group of the support-bound oligonucleotide.
Further reading
- Comprehensive Natural Products Chemistry, Volume 7: DNA and Aspects of Molecular Biology. Kool, Eric T.; Editor. Neth. (1999), 733 pp. Publisher: (Elsevier, Amsterdam, Neth.)
- Beaucage, S. L.; Iyer, R. P. Advances in the synthesis of oligonucleotides by the phosphoramidite approach. Tetrahedron 1992, 48, 2223–2311.
- Beaucage, S. L.; Iyer, R. P. The functionalization of oligonucleotides via phosphoramidite derivatives. Tetrahedron 1993, 49, 1925–1963.
- Beaucage, S. L.; Iyer, R. P. The synthesis of modified oligonucleotides by the phosphoramidite approach and their applications. Tetrahedron 1993, 49, 6123–6194.
- Beaucage, S L. Oligodeoxyribonucleotides synthesis. Phosphoramidite approach. Methods in Molecular Biology (Totowa, NJ, United States) (1993), 20 (Protocols for Oligonucleotides and Analogs), 33–61.
- Reese, C. B. The chemical synthesis of oligo- and poly-nucleotides: a personal commentary. Tetrahedron 2002, 58, 8893–8920.
- Brown T., Brown D. J. S. 1991. In Oligonucleotides and Analogues. A Practical Approach, ed. F Eckstein, pp. 1 – 24. Oxford: IRL
See also
- Oligonucleotide synthesisOligonucleotide synthesisOligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acids with defined chemical structure . The technique is extremely useful in current laboratory practice because it provides a rapid and inexpensive access to custom-made oligonucleotides of the desired...
- DNA synthesisDNA synthesisDNA synthesis commonly refers to:*DNA replication - DNA biosynthesis *Polymerase chain reaction - enzymatic DNA synthesis *Oligonucleotide synthesis - chemical synthesis of nucleic acids...
- Nucleic acid analoguesNucleic acid analoguesNucleic acid analogues are compounds structurally similar to naturally occurring RNA and DNA, used in medicine and in molecular biology research....

