Phosphazene
Encyclopedia
Phosphazenes are a class of chemical compound
s in which a phosphorus
atom
is covalently linked
to a nitrogen
atom
by a double bond
and to three other atoms or radicals by single bonds. While other substitutions produce relatively persistent compounds, in organic synthesis
the term largely refers to species with three amino substituents bound to phosphorus. The compounds are unusually stable examples of the phosphorane
class of molecules and have a remarkable proton affinity
. As such, they are one of the eminent examples of neutral, organic superbases
. Two examples are hexachlorocyclotriphosphazene and bis(triphenylphosphine)iminium chloride
. Phosphazenes are also known as iminophosphoranes and phosphine imides.
The corresponding polymer
s are polyphosphazene
s.
than regular amine
or amidine bases such as Hünig's base or DBU
. Protonation takes place at a doubly bonded nitrogen atom. Related to phosphazene bases are the proazaphosphatrane bases, which have a saturated P(NR)3 structure and protonate at phosphorus.
Though the simplest phosphazene superbase, P1-Me, was first synthesized in 1975, chemists assumed that the compounds were highly unstable, like their alkyl-substituted derivatives. The species was regarded at that time as little more than an academic curiosity.
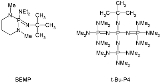
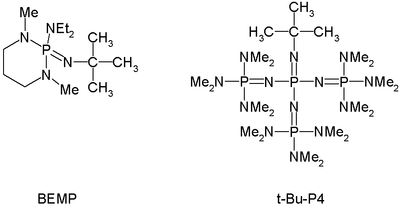 By now phosphazene bases are established reagents in organic synthesis. Perhaps the best known phosphazene bases are BEMP with an acetonitrile
By now phosphazene bases are established reagents in organic synthesis. Perhaps the best known phosphazene bases are BEMP with an acetonitrile
pKa
of the conjugate acid
of 27.6 and the phosphorimidic triamide t-Bu-P4 (pKBH+ = 42.7) also known as Schwessinger base after one of its inventors.
In one application t-Bu-P4 is employed in a nucleophilic addition
converting the pivalaldehyde to the alcohol
:
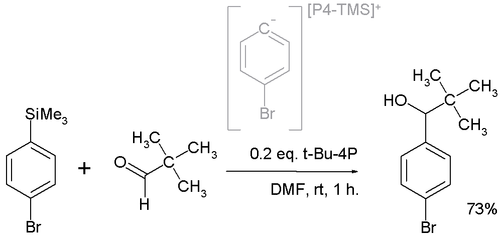 The active nucleophile
The active nucleophile
is believed to be a highly reactive phosphazenium species with full negative charge on the arene sp2 carbon.
Besides organic synthesis, phosphazene bases are used as basic titrants in non-aqueous acid-base titration
. Their advantages for this are: they are very strong bases in many solvents and their conjugate acids are inert and non-HBD cations.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s in which a phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
is covalently linked
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
to a nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
by a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
and to three other atoms or radicals by single bonds. While other substitutions produce relatively persistent compounds, in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
the term largely refers to species with three amino substituents bound to phosphorus. The compounds are unusually stable examples of the phosphorane
Phosphorane
A phosphorane is a functional group in organophosphorus chemistry with pentavalent phosphorus. It has the general formula PR5. The parent hydride compound is the unstable molecule PH5...
class of molecules and have a remarkable proton affinity
Proton affinity
The proton affinity, Epa, of an anion or of a neutral atom or molecule is a measure of its gas-phase basicity. It is the energy released in the following reactions:-Acid/base chemistry:...
. As such, they are one of the eminent examples of neutral, organic superbases
Superbase
In chemistry, a superbase is an extremely strong base, that is a compound that has a high affinity for protons. Hydroxide ion is the strongest base possible in aqueous solutions, but bases exist with pKb's well outside of the aqueous range. Such bases are valuable in organic synthesis and are...
. Two examples are hexachlorocyclotriphosphazene and bis(triphenylphosphine)iminium chloride
Bis(triphenylphosphine)iminium chloride
Bisiminium chloride is the chemical compound with the formula [3P)2N]Cl, often written [2N]Cl and abbreviated PPNCl. This colorless salt is a source of the PPN+ cation, which is used to isolate reactive anions...
. Phosphazenes are also known as iminophosphoranes and phosphine imides.
The corresponding polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s are polyphosphazene
Polyphosphazene
Polyphosphazenes are a class of inorganic polymers with the repeat unit . The substituents R,R' are usually alkoxy, amino, , or halogens ....
s.
Phosphazene bases
Phosphazene bases are strong non-metallic non-ionic and low-nucleophilic bases. They are stronger basesBase (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
than regular amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
or amidine bases such as Hünig's base or DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
. Protonation takes place at a doubly bonded nitrogen atom. Related to phosphazene bases are the proazaphosphatrane bases, which have a saturated P(NR)3 structure and protonate at phosphorus.
Though the simplest phosphazene superbase, P1-Me, was first synthesized in 1975, chemists assumed that the compounds were highly unstable, like their alkyl-substituted derivatives. The species was regarded at that time as little more than an academic curiosity.

Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of the conjugate acid
Conjugate acid
Within the Brønsted–Lowry acid-base theory , a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases, or donates, a proton in the forward chemical...
of 27.6 and the phosphorimidic triamide t-Bu-P4 (pKBH+ = 42.7) also known as Schwessinger base after one of its inventors.
In one application t-Bu-P4 is employed in a nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
converting the pivalaldehyde to the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
:

Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
is believed to be a highly reactive phosphazenium species with full negative charge on the arene sp2 carbon.
Besides organic synthesis, phosphazene bases are used as basic titrants in non-aqueous acid-base titration
Acid-base titration
An acid-base titration is the determination of the concentration of an acid or base by exactly neutralizing the acid/base with an acid or base of known concentration. This allows for quantitative analysis of the concentration of an unknown acid or base solution...
. Their advantages for this are: they are very strong bases in many solvents and their conjugate acids are inert and non-HBD cations.

