Phase Boundary Catalysis
Encyclopedia
In chemistry
, phase-boundary catalysis (PBC) is a type of heterogeneous catalytic system which facilitates the chemical reaction
of a particular chemical component in immiscible phase react on a catalytic active site located at phase boundary. The chemical component is soluble in one phase but insoluble in the other. The catalyst for PBC has been designed in which the external part of the zeolite
is hydrophobic, internally it is usually hydrophilic, notwithstanding to polar nature of some reactants. In this sense, the medium environment in this system is close to that of an enzyme
. The major difference between this system and enzyme
is lattice flexibility. The lattice of zeolite
is rigid, whereas the enzyme
is flexible.
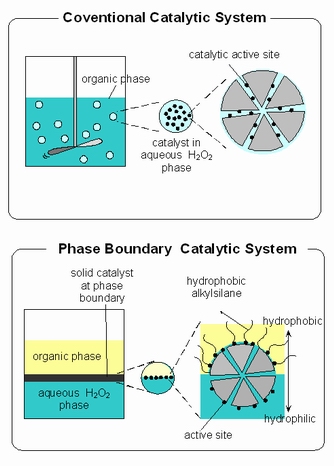
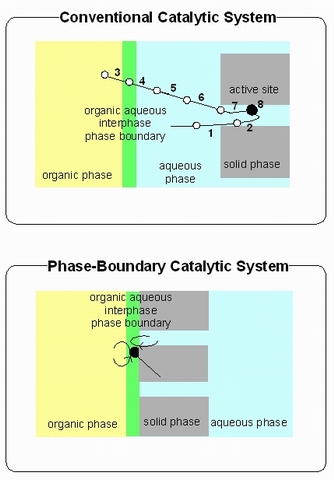
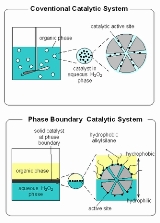
 Figure 1 shows schematic representation of design of phase-boundary catalytic (PBC) system and its comparison with conventional catalytic system. The PBC is useful primarily for performing reaction at the interface of aqueous phase and organic substrate phases. PBC is needed because the immiscibility of aqueous phase and organic substrate. The name phase-boundary catalysis does what it says; the catalyst acts as a catalyst at the interphase between the aqueous and organic phases as shown in Figure 1. The reaction medium of phase-boundary catalysis system for the catalytic reaction of immiscible aqueous and organic phases consist of three phases; an organic liquid phase, containing most of the substrate, an aqueous liquid phase containing most of the substrate in aqueous phase and the solid catalyst. The two liquid phases are almost completely insoluble in one another.
Figure 1 shows schematic representation of design of phase-boundary catalytic (PBC) system and its comparison with conventional catalytic system. The PBC is useful primarily for performing reaction at the interface of aqueous phase and organic substrate phases. PBC is needed because the immiscibility of aqueous phase and organic substrate. The name phase-boundary catalysis does what it says; the catalyst acts as a catalyst at the interphase between the aqueous and organic phases as shown in Figure 1. The reaction medium of phase-boundary catalysis system for the catalytic reaction of immiscible aqueous and organic phases consist of three phases; an organic liquid phase, containing most of the substrate, an aqueous liquid phase containing most of the substrate in aqueous phase and the solid catalyst. The two liquid phases are almost completely insoluble in one another.
In case of conventional catalytic system (see Figure 1);
1. transfer of aqueous phase from organic phase to the external surface
of solid catalyst;
2. transfer of aqueous phase inside the pore volume of solid catalyst;
3. transfer of the substrate from aqueous phase to the interphase between aqueous and organic phases;
4. transfer of the substrate from the interphase to the aqueous phase;
5. mixing and diffusion of the substrate in the aqueous phase;
6. transfer of the substrate from the aqueous phase to the external
surface of solid catalyst;
7. transfer of the substrate inside the pore volume of the solid catalyst; and
8. catalytic reaction (adsorption
, chemical reaction
and desorption
).
It was reported that without vigorous stirring, no reactivity of the catalyst was observed in conventional catalytic system.[1] [2] [3] [4] [5] As proposed in Figure 2, it is clear that stirring and mass transfer from organic to aqueous phase and vice-versa are required for conventional catalytic system. In the PBC (Figure 2), the stirring is not required because the mass transfer is not rate determining step in this catalytic system. It is already demonstrated that this system works for alkene epoxidation without stirring or the addition of a co-solvent to drive liquid–liquid phase transfer.[1] [2] [3] The active site located on the external surface of the zeolite particle were dominantly effective for the observed phase boundary catalytic system.[4]
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, phase-boundary catalysis (PBC) is a type of heterogeneous catalytic system which facilitates the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of a particular chemical component in immiscible phase react on a catalytic active site located at phase boundary. The chemical component is soluble in one phase but insoluble in the other. The catalyst for PBC has been designed in which the external part of the zeolite
Zeolite
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents. The term zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that upon rapidly heating the material stilbite, it produced large amounts of steam from water that...
is hydrophobic, internally it is usually hydrophilic, notwithstanding to polar nature of some reactants. In this sense, the medium environment in this system is close to that of an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
. The major difference between this system and enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
is lattice flexibility. The lattice of zeolite
Zeolite
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents. The term zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that upon rapidly heating the material stilbite, it produced large amounts of steam from water that...
is rigid, whereas the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
is flexible.
Design of phase-boundary catalyst



In case of conventional catalytic system (see Figure 1);
- When the reaction mixture is vigorously stirred, an apparently homogeneous emulsion is obtained, which segregates very rapidly into two liquid phases when the agitation ceases. Segregation occurs by formation of organic bubbles in the emulsion which move downwards to form the aqueous phase, indicating that emulsion consists of dispersed particles of the aqueous phase in the organic phase.
- Due to the triphasic reactions conditions, the overall reaction between aqueous phase and organic phase substrates on solid catalyst requires different transfer processes. The following steps, which are schematically represented in Figure 2 are involved:
1. transfer of aqueous phase from organic phase to the external surface
of solid catalyst;
2. transfer of aqueous phase inside the pore volume of solid catalyst;
3. transfer of the substrate from aqueous phase to the interphase between aqueous and organic phases;
4. transfer of the substrate from the interphase to the aqueous phase;
5. mixing and diffusion of the substrate in the aqueous phase;
6. transfer of the substrate from the aqueous phase to the external
surface of solid catalyst;
7. transfer of the substrate inside the pore volume of the solid catalyst; and
8. catalytic reaction (adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
, chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
and desorption
Desorption
Desorption is a phenomenon whereby a substance is released from or through a surface. The process is the opposite of sorption . This occurs in a system being in the state of sorption equilibrium between bulk phase and an adsorbing surface...
).
It was reported that without vigorous stirring, no reactivity of the catalyst was observed in conventional catalytic system.[1] [2] [3] [4] [5] As proposed in Figure 2, it is clear that stirring and mass transfer from organic to aqueous phase and vice-versa are required for conventional catalytic system. In the PBC (Figure 2), the stirring is not required because the mass transfer is not rate determining step in this catalytic system. It is already demonstrated that this system works for alkene epoxidation without stirring or the addition of a co-solvent to drive liquid–liquid phase transfer.[1] [2] [3] The active site located on the external surface of the zeolite particle were dominantly effective for the observed phase boundary catalytic system.[4]

