
Persistent carbene
Encyclopedia

Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
demonstrating particular stability. The best-known examples are diaminocarbenes with the general formula (R2N)2C:, where the 'R's are various functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s. The groups can be bridged so that the carbon with unfilled orbitals is part of an heterocycle
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
, such as imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
or triazole
Triazole
Triazole refers to either one of a pair of isomeric chemical compounds with molecular formula C2H3N3, having a five-membered ring of two carbon atoms and three nitrogen atoms.The two isomers are:*1,2,3-Triazole 100px*1,2,4-Triazole 100px...
.
Carbenes have long been known as very reactive and short lived molecules that could not be isolated, and were usually studied by observing the reactions they undergo. Stable carbenes had been proposed to exist by R. Breslow
Ronald Breslow
Ronald C. D. Breslow is an American chemist from Rahway, New Jersey. He is currently University Professor at Columbia University, where he is based in the Department of Chemistry and affiliated with the Departments of Biological Sciences and Pharmacology; he has also been on the faculty of its...
in 1957, and the first examples of stable carbenes coordinated to metal atoms were synthesized by H.-W. Wanzlick
Hans-Werner Wanzlick
Hans-Werner Wanzlick was a professor of chemistry at the Berlin Technical University. He is notable for work on persistent carbenes and for proposing the Wanzlick equilibrium between saturated imidazolin-2-ylidenes and their dimers — which he called "das doppelte Lottchen", after a 1949 novel by...
and collaborators. The isolation of a stable liquid dicarbene was reported in 1989 by G. Bertrand
Guy Bertrand (chemist)
Guy Bertrand is a chemistry professor at the University of California, Riverside.Bertrand obtained his B.Sc. from the University of Montpellier in 1975 and his Ph.D. from the Paul Sabatier University, Toulouse, in 1979...
and others. In 1991, the group of A. Arduengo
Anthony Joseph Arduengo III
Anthony Joseph Arduengo, III is the Saxon Professor of Chemistry at the University of Alabama and an adjunct professor at the Institute for Inorganic Chemistry of Braunschweig University of Technology in Germany...
reported the synthesis of a stable, isolated, crystalline carbene.
Persistent carbenes are still fairly reactive substances, and many will undergo dimerisation, sometimes reversibly.
Persistent carbenes can exist in the singlet state or the triplet state
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
, with the singlet state carbenes being more stable. The relative stability of these compounds is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
in the absence of moisture and (in most cases) oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, and can be isolated and indefinitely stored. Others are not thermodynamically stable and will dimerise slowly over days. The less stable triplet state carbenes have half-lives measured in seconds, and therefore can be observed but not stored.
Conjectures
In 1957, Breslow proposed that a relatively stable nucleophilic carbene, a thiazol-2-ylidene derivative, was involved in the catalytic cycleCatalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
of vitamin B1 (thiamine) that yields furoin
Furoin
Furoin or 1,2-di-2-hydroxyethanone is an organic compound with formula C10H8O4. It can be produced from furfural by the catalytic action of cyanide ions.-Reactions:...
from furfural
Furfural
Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word , meaning bran, referring to its usual source....
. In this cycle, the vitamin's thiazolium ring exchanges an hydrogen atom (attached to carbon 2 of the ring) for a furfural residue. Through a deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
exchange experiment, Breslow demonstrated that under standard reaction conditions (in deuterated water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
) the C2-proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
was rapidly exchanged for a deuteron in a statistical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
.
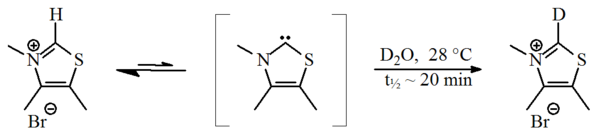
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
.
In 1960, H.-P. Wanzlick and co-workers conjectured that carbenes derived from dihydroimidazol-2-ylidene
Dihydroimidazol-2-ylidene
Dihydroimidazol-2-ylidene is a hypothetical organic compound with formula C3H6N2. It would be a heterocyclic compound, formally derived from imidazolidine with two hydrogen atoms removed from carbon number 2, leaving two vacant chemical bonds — which makes it a carbene.Although carbenes in general...
were produced by vacuum pyrolysis of the corresponding 2-trichloromethyl dihydroimidazole compounds with the loss of chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
. They conjectured that the carbene existed in an unfavourable equilibrium with its corresponding dimer (a tetraaminoethylene
Tetraaminoethylene
Tetraaminoethylene is a hypothetical organic compound with formula C2N4H8 or 2C=C2. Like all geminal polyamines, this compound has never been synthesised and is believed to be extremely unstable....
derivative), in the so-called Wanzlick equilibrium
Wanzlick equilibrium
The Wanzlick equilibrium is a chemical equilibrium between a relatively stable carbene compound and its dimer.-Original conjecture:In 1960, H.-W. Wanzlick and E...
. This conjecture was challenged by Lemal and co-workers in 1964, who presented evidence that the dimer did not dissociate; and also by Winberg in 1965. However, subsequent experiments by M. Denk
Michael K. Denk
Michael K. Denk is a Professor of chemistry at the University of Guelph, Ontario.Michael Denk obtained his M.Sc. at the Ludwig Maximilian University in Munich, Germany . He got his Ph.D. at the Technical University of Munich , advised by W. Herrmann, with a dissertation on cyclic metalloamides...
, W. A. Herrmann and others have confirmed the reality of the equilibrium, in specific circumstances.
First synthesis
In 1970, Wanzlick's group prepared the first imidazol-2-ylidene carbene, by the deprotonation of an imidazolium salt. Wanzlick, as well as Hoffmann, believed that these imidazole-based carbenes, with a 4n+2Hückel's rule
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931...
π-electron ring system, should be more stable than the 4,5-dihydro analogues, due to Hückel-type aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
. The carbenes were not isolated, but obtained as coordination compounds with mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
and isothiocyanate
Isothiocyanate
Isothiocyanate is the chemical group –N=C=S, formed by substituting sulfur for oxygen in the isocyanate group. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also...
:
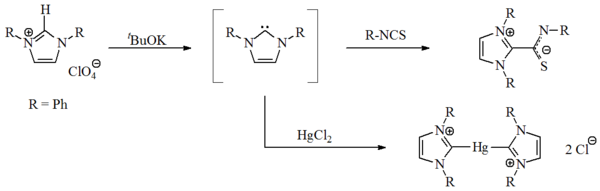
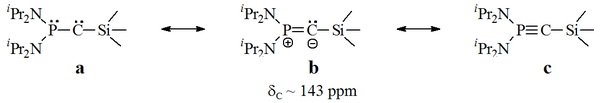
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
reactivity. An X-ray structure of this molecule has not been obtained and at the time of publication some doubt remained as to their exact carbenic nature.
In 1991, a stable, isolated, and crystalline dicarbene, which can be represented as a carbene or a nitrogen carbon ylide, was obtained by A. Arduengo and co-workers, by deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of an imidazolium chloride with a strong base:
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
spectrum at 211 ppm for the carbenic atom. The X-ray structure revealed longer N–C bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s in the ring of the carbene than in the parent imidazolium compound, indicating that there was very little double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
character to these bonds.
The first air-stable ylidic carbene, a chlorinated member of the imidazol-2-ylidene family, was obtained in 1997.
In 2000, Bertrand obtained additional carbenes of the phosphanyl type, including (phosphanyl)(trifluoromethyl)carbene, stable in solution at – 30° and a moderately stable (amino)(aryl)carbene with only one heteroatom adjacent to the carbenic atom.
Understanding their stability

Thermodynamic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity...
.

Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
character to that system, was important for the carbene's stability. This conjecture was disproved in 1995 by Arduengo's group, who obtained a derivative dihydroimidazol-2-ylidene
Dihydroimidazol-2-ylidene
Dihydroimidazol-2-ylidene is a hypothetical organic compound with formula C3H6N2. It would be a heterocyclic compound, formally derived from imidazolidine with two hydrogen atoms removed from carbon number 2, leaving two vacant chemical bonds — which makes it a carbene.Although carbenes in general...
without that double bond. The thermodynamical stability in this compound, and the role of steric protection in preventing dimerisation, has been a topic of some dispute.

Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
character could be measured, and the ylidic
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
nature of this carbene could be determined. Like the cyclic diaminocarbenes, unhindered variants tend to dimerise.
Until 1997, all stable carbenes known had two nitrogen atoms bound to the carbenic atom. This pattern was broken in 1997–1998 with the sythesis of a thiazol-2-ylidene derivative by Arduengo's group and an aminothiocarbene and an aminooxycarbene by Alder's group. In these stable compounds, the carbenic atom lies between a nitrogen atom and either a sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
or oxygen atom:
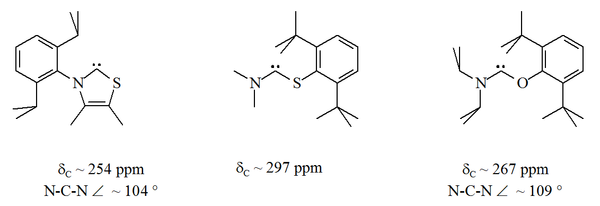
A more radical development was the synthesis in 2006 of bis(diisopropylamino)cyclopropenylidene by Bertrand's group. In this compound, stable at room temperature, the carbene atom is connected to two carbon atoms, in a three-member ring that retains the aromaticity and geometry of the cyclopropenylidene
Cyclopropenylidene
Cylopropenylidene, or c-C3H2 belongs to a highly reactive class of organic molecules known as carbenes. Due to its reactivity, cyclopropenylidene is only seen terrestrially in the laboratory. However, it is found in significant concentrations in the interstellar medium due to the extreme environment...
ring. This example demonstrated that the presence of heteroatoms next to the carbene is not necessary for stability, either.
Classes of stable carbenes
The following are examples of the classes of stable carbenes isolated to date:Imidazol-2-ylidenes
The first stable carbenes to be isolated were based on an imidazoleImidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
ring, with the hydrogen in carbon 2 of the ring (between the two nitrogen atoms) removed, and other hydrogens replaced by various groups. These imidazol-2-ylidenes are still the most stable and the most well studied and understood family of persistent carbenes.
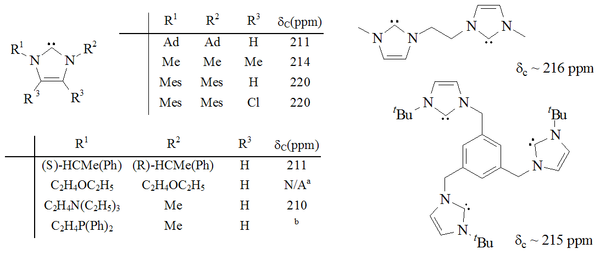
A considerable range of imidazol-2-ylidenes have been synthesised, including those in which the 1,3-positions have been functionalised with alkyl, aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
, alkyloxy, alkylamino, alkylphosphino and even chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
substituents:

Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
atoms for the two hydrogens at ring positions 4 and 5 yielded the first air-stable carbene. Its extra stability probably results from the electron-withdrawing effect of the chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
atoms, which must reduce the electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
on the carbon atom bearing the lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
, via induction
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
through the sigma-backbone.
Molecules containing two and even three imidazol-2-ylidene groups have also been synthesised.
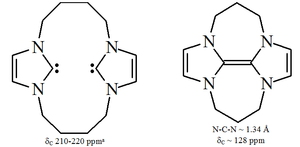
Imidazole-based carbenes are thermodynamically stable and generally have diagnostic 13C NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
chemical shift values between 210–230 ppm for the carbenic carbon. Typically, X-ray structures of these molecules show N-C-N bond angles of 101–102°.
Triazol-5-ylidenes
Another family of persistent carbenes are based on the 1,2,4-triazole1,2,4-Triazole
1,2,4-Triazole is one of a pair of isomeric chemical compounds with molecular formula C2H3N3, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,4-Triazole is a basic aromatic heterocycle...
ring, with the unfilled orbitals in carbon 5 of this ring. The triazol-5-ylidenes pictured below were first prepared by Enders and co-workers by vacuum pyrolysis through loss of methanol from 2-methoxytriazoles. Only a limited range of these molecules have been reported, with the triphenyl substituted molecule being commercially available.
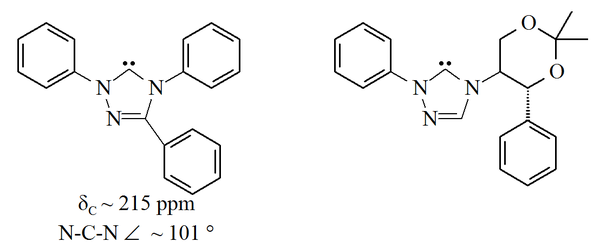
Triazole
Triazole refers to either one of a pair of isomeric chemical compounds with molecular formula C2H3N3, having a five-membered ring of two carbon atoms and three nitrogen atoms.The two isomers are:*1,2,3-Triazole 100px*1,2,4-Triazole 100px...
-based carbenes are thermodynamically stable and have diagnostic 13C NMR chemical shift values between 210–220 ppm for the carbenic carbon. The X-ray structure of the triphenyl substituted carbene above shows an N-C-N bond angle of ca. 101°. The 5-methoxytriazole precursor to this carbene was made by the treatment of a triazolium salt with sodium methoxide, which attacks as a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. This may indicate that these carbenes are less aromatic than imidazol-2-ylidenes, as the imidazolium precursors do not react with nucleophiles due to the resultant loss of aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
.
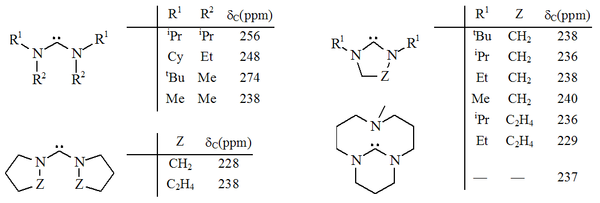
Other diaminocarbenes
The two families above can be seen as special cases of a broader class of compounds which have a carbenic atom bridging two nitrogen atoms. A range of such diaminocarbenes have been prepared principally by Roger AlderRoger Alder
Roger William Alder, FRS is an Emeritus Professor of organic chemistry at the University of Bristol.His research involves the study of novel compounds with unusual properties, such as proton sponges and stable carbenes.-External links:*...
's research group. In some of these compounds, the N-C-N unit is a member of a 5 or 6 membered non-aromatic ring, including a bicyclic example. In other examples, the adjacent nitrogens are connected only through the carbenic atom, and may or may not be part of separate rings.
Wanzlick equilibrium
The Wanzlick equilibrium is a chemical equilibrium between a relatively stable carbene compound and its dimer.-Original conjecture:In 1960, H.-W. Wanzlick and E...
).
Diaminocarbenes have diagnostic 13C NMR chemical shift values between 230–270 ppm for the carbenic atom. The X-ray structure of dihydroimidazole-2-ylidene shows a N-C-N bond angle of ca. 106°, whilst the angle of the acyclic carbene is 121°, both greater than those seen for imidazol-2-ylidenes.
Heteroamino carbenes
There exist several variants of the stable carbenes above where one of the nitrogen atoms adjacent to the carbene center (the α nitrogens) has been replaced by an alternative heteroatom, such as oxygen, sulfur, or phosphorusPhosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
.:

Thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS...
. A thiazole based carbene (analogous to the carbene postulated by Breslow) has been prepared and characterised by X-ray crystallography. Other non-aromatic aminocarbenes with O, S and P atoms adjacent (i.e. alpha) to the carbene centre have been prepared, e.g. thio- and oxy-iminium based carbenes have been characterised by X-ray crystallography.
Since oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and sulfur are divalent
Divalent
In chemistry, a divalent ion or molecule has a valence of two and thus can form two bonds with other ions or molecules. An older term for divalent is bivalent....
, steric protection of the carbenic centre is limited especially when the N-C-X unit is part of a ring. These acyclic carbenes have diagnostic 13C NMR chemical shift values between 250–300 ppm for the carbenic carbon, further downfield than any other types of stable carbene. X-ray structures have show N-C-X bond angles of ca. 104 ° and 109 ° respectively.
Non-amino carbenes
Carbenes that formally derive from imidazole-2-ylidenes by substitution of sulfur, oxygen, or other chalcogenChalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
s for both α-nitrogens are expected to be unstable, as they have the potential to dissociate into an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
(R1C≡CR2) and a carbon dichalcogenide
Chalcogenide
A chalcogenide is a chemical compound consisting of at least one chalcogen ion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term is more commonly reserved for sulfides, selenides, and tellurides, rather than...
(X1=C=X2).
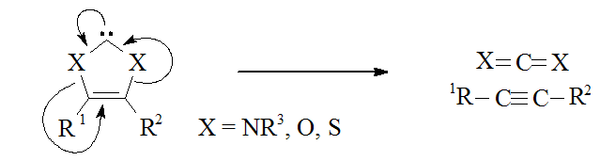
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
CS2 with electron deficient acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
derivatives gives transient 1,3-dithiolium carbenes (i.e. where X1 = X2 = S) which then dimerise. Thus it is possible that the reverse of this process might be occurring in similar carbenes.
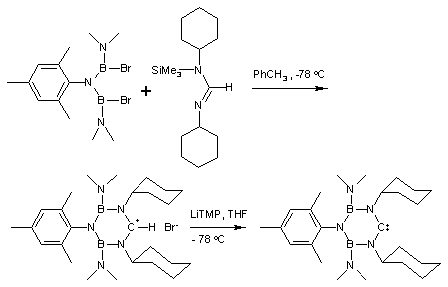
Bertrand's carbenes
In Bertrand's persistent carbenes, the unfilled carbon is bonded to a phosphorusPhosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and a silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
. However, these compounds seem to exhibit some alkynic properties, and when published the exact carbenic nature of these red oils was in debate.
Other nucleophilic carbenes
One stable N-heterocyclic carbene has a structure analogous to borazineBorazine
Borazine is an inorganic compound with the chemical formula 33. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene...
with one boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
atom replaced by methylene. This results in a planar 6 electron compound.
Cyclopropenylidenes
Another family of carbenes is based on a cyclopropenylideneCyclopropenylidene
Cylopropenylidene, or c-C3H2 belongs to a highly reactive class of organic molecules known as carbenes. Due to its reactivity, cyclopropenylidene is only seen terrestrially in the laboratory. However, it is found in significant concentrations in the interstellar medium due to the extreme environment...
core, a three-carbon ring with a double bond between the two atoms adjacent to the carbenic one. This family is exemplified by bis(diisopropylamino)cyclopropenylidene
Triplet state carbenes
In 2001, Hideo Tomioka and his associates were able to produce a comparatively stable triplet carbene (bis(9-anthryl)carbene, with a half-life of 19 minutes), by taking advantage of resonanceResonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
.
In 2006 the same group reported a triplet carbene with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 40 minutes. This carbene is prepared by a photochemical
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
decomposition
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
of a diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
with expulsion of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
gas at a wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
of 300 nanometers in benzene.
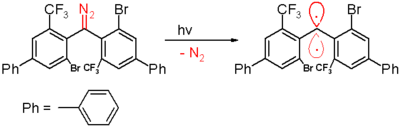
Benzophenone
Benzophenone is the organic compound with the formula 2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.-Uses:...
and the diphenylmethane compound is formed when it is trapped by 1,4-cyclohexadiene
1,4-Cyclohexadiene
1,4-Cyclohexadiene is a highly flammable cycloalkene that occurs as a colorless clear liquid.1,4-Cyclohexadiene and related compounds may be prepared from benzene using lithium or sodium in liquid ammonia, this process being known as a Birch reduction. However 1,4-cyclohexadiene is easily oxidised...
. As with the other carbenes this species contains large bulky substituents, namely bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
and the trifluoromethyl groups, that shield the carbene and prevent or slow down the process of dimerisation to a 1,1,2,2-tetra(phenyl)alkene.
Based on computer simulations
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
, the distance
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
of the divalent carbon atom to its neighbours is claimed to be 138 picometers with a bond angle of 158.8°. The planes of the phenyl groups are almost at right angles to each other (the dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
being 85.7°).
Basicity and nucleophilicity
The nucleophilicity and basicity of imidazol-2-ylidenes have been studied by Alder et al. who revealed that these molecules are strong bases, having a pKaPKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of ca. 24 for the conjugate acid in dimethyl sulfoxide
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
(DMSO):

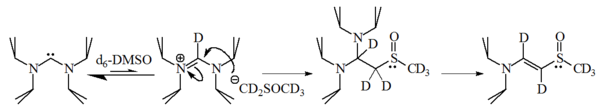
1-bromohexane
1-Bromohexane is a liquid organohalide with structural formula Br-CH2-CH2-CH2-CH2-CH2-CH3. Its refractive index is 1.4478 .It is used to manufacture pharmaceuticals and organic chemicals.- External links :*...
gave 90% of the 2-substituted adduct, with only 10% of the corresponding alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
, indicating that these molecules are also reasonably nucleophilic.
Dimerisation
At one time, stable carbenes were thought to reversibly dimerise through the so-called Wanzlick equilibriumWanzlick equilibrium
The Wanzlick equilibrium is a chemical equilibrium between a relatively stable carbene compound and its dimer.-Original conjecture:In 1960, H.-W. Wanzlick and E...
. However, imidazol-2-ylidenes and triazol-5-ylidenes are thermodynamically stable and do not dimerise, and have been stored in solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
in the absence of water and air for years. This is presumably due to the aromatic nature of these carbenes, which is lost upon dimerisation. In fact imidazol-2-ylidenes are so thermodynamically stable that only in highly constrained conditions are these carbenes forced to dimerise.
Chen and Taton made a doubly tethered diimidazol-2-ylidene by deprotonating the respective diimidazolium salt. Only the deprotonation of the doubly tethered diimidazolium salt with the shorter methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
(-CH2-) linkage resulted in the dicarbene dimer:
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s on the carbenic carbon would be forced into close proximity. Presumably the resulting repulsive electrostatic interactions would have a significant destabilising effect. To avoid this electronic interaction, the carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
units dimerise.
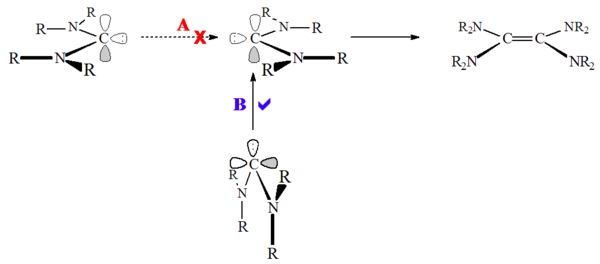
On the other hand, heteroamino carbenes (e.g. R2N-C:-OR or R2N-C:-SR) and non-aromatic carbenes such as diaminocarbenes (e.g. R2N-C:-NR2) have been shown to dimerise, albeit quite slowly. This has been presumed to be due to the high barrier to singlet state dimerisation:
Formamidinium
Formamidinium cations are positively charged organic chemical compounds which can be represented by the chemical formula [R2N-CH=NR2]+.-Chemical applications:...
salts, a protonated precursor species. Accordingly, this reaction can be acid catalysed. This reaction occurs because unlike imidazolium based carbenes, there is no loss of aromaticity in protonation of the carbene.
Unlike the dimerisation of triplet state
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
carbenes, these singlet state carbenes do not approach head to head ("least motion"), but rather the carbene lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
attacks the empty carbon p-orbital ("non-least motion"). Carbene dimerisation can also be acid or metal catalysed, and so care must be taken when determining if the carbene is undergoing true dimerisation.
Reactivity
The chemistry of stable carbenes has not been fully explored. However, Enders et al. have performed a range of organic reactions involving a triazol-5-ylidene. These reactions are outlined below and may be considered as a model for other carbenes.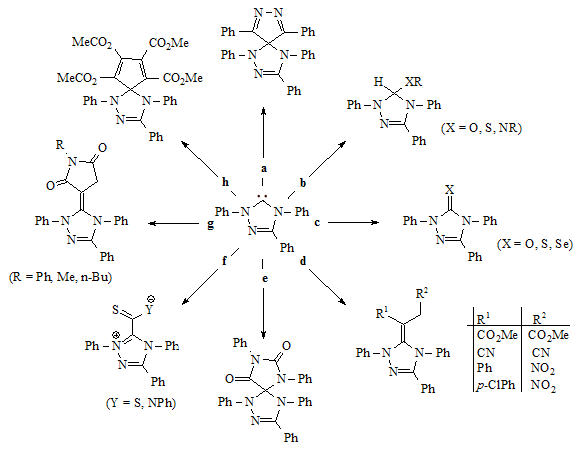
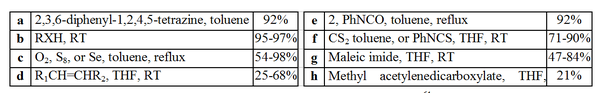
Insertion reaction
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:...
s (b), addition reaction
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
s (c), [2+1] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s (d, g and h), [4+1] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s (a) as well as simple deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
s. The insertion reaction
Insertion reaction
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:...
s (b) probably proceed via deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
, resulting in the generation of a nucleophile (-XR) which can attack the generated salt giving the impression of a H-X insertion.
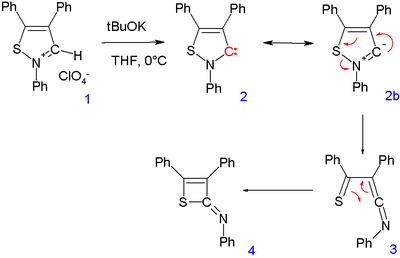
Care must be taken to check that a stable carbene is truly stable. The discovery of a stable isothiazole
Isothiazole
An isothiazole is a type of organic compound containing a five-membered aromatic ring that consists of three carbon atoms, one nitrogen atom, and one sulfur atom. Isothiazole is a member of a class of compounds known as azoles...
carbene (2) from an isothiazolium perchlorate (1) by one research group was questioned by another group who were only able to isolate 2-imino-2H-thiete (4). The intermediate 3 was proposed through a rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
. This carbene is no longer considered stable.
Carbene complexation
Imidazol-2-ylidenes, triazol-5-ylidenes (and less so, diaminocarbenes) have been shown to co-ordinate to a plethora of elements, from alkali metals, main group elementMain group element
In chemistry and atomic physics, main group elements are elements in groups whose lightest members are represented by helium, lithium,...
s, transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s and even lanthanides and actinides. A periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
of elements gives some idea of the complexes which have been prepared, and in many cases these have been identified by single crystal X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
.
| Group Periodic table group In chemistry, a group is a vertical column in the periodic table of the chemical elements. There are 18 groups in the standard periodic table, including the d-block elements, but excluding the f-block elements.... → |
1 Alkali metal The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium... |
2 Alkaline earth metal The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and... |
3 Group 3 element The group 3 elements are a group of chemical elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group... |
4 Group 4 element The Group 4 elements are a group of chemical elements in the periodic table. In the modern IUPAC nomenclature, Group 4 of the periodic table contains titanium , zirconium , hafnium and rutherfordium . This group lies in the d-block of the periodic table... |
5 Group 5 element A Group 5 element is a chemical element in the fifth group in the periodic table. In the modern IUPAC nomenclature, Group 5 of the periodic table contains vanadium , niobium , tantalum and dubnium . This group lies in the d-block of the periodic table... |
6 Group 6 element A Group 6 element is one in the series of elements in group 6 in the periodic table, which consists of the transition metals chromium , molybdenum , tungsten , and seaborgium .... |
7 Group 7 element A Group 7 element is one in the series of elements in group 7 in the periodic table, which consists of manganese , technetium , rhenium , and bohrium... |
8 Group 8 element A Group 8 element is one in the series of elements in group 8 in the periodic table, which consists of the transition metals iron , ruthenium , osmium and hassium .... |
9 Group 9 element In modern IUPAC nomenclature, Group 9 of the periodic table contains the elements cobalt , rhodium , iridium , and meitnerium . These are all d-block transition metals... |
10 Group 10 element A Group 10 element is one in the series of elements in group 10 in the periodic table, which consists of the transition metals nickel , palladium , platinum , and darmstadtium .... |
11 Group 11 element A Group 11 element is one in the series of elements in group 11 in the periodic table, consisting of transition metals which are the traditional coinage metals of copper , silver , and gold... |
12 Group 12 element A group 12 element is one of the elements in group 12 in the periodic table. This includes zinc , cadmium and mercury . The further inclusion of copernicium in group 12 is supported by recent experiments on individual Cn atoms... |
13 Boron group The boron group is the series of elements in group 13 of the periodic table, comprising boron , aluminium , gallium , indium , thallium , and ununtrium . The elements in the boron group are characterized by having three electrons in their outer energy levels... |
14 Carbon group The carbon group is a periodic table group consisting of carbon , silicon , germanium , tin , lead , and ununquadium .... |
15 Nitrogen group The nitrogen group is a periodic table group consisting of nitrogen , phosphorus , arsenic , antimony , bismuth and ununpentium .... |
16 Chalcogen The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family... |
17 Halogen The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine... |
18 Noble gas The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity... |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓ Period Periodic table period In the periodic table of the elements, elements are arranged in a series of rows so that those with similar properties appear in vertical columns. Elements of the same period have the same number of electron shells; with each group across a period, the elements have one more proton and electron... |
||||||||||||||||||||
| 1 Period 1 element A period 1 element is one of the chemical elements in the first row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical... |
||||||||||||||||||||
| 2 Period 2 element A period 2 element is one of the chemical elements in the second row of the periodic table. The periodic table is laid out in rows to illustrate recurring trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to... |
||||||||||||||||||||
| 3 Period 3 element A period 3 element is one of the chemical elements in the third row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical... |
||||||||||||||||||||
| 4 Period 4 element A period 4 element is one of the chemical elements in the fourth row of the periodic table of the elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour... |
||||||||||||||||||||
| 5 Period 5 element A period 5 element is one of the chemical elements in the fifth row of the periodic table of the elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour... |
||||||||||||||||||||
| 6 Period 6 element A period 6 element is one of the chemical elements in the sixth row of the periodic table of the elements, including the lanthanides... |
||||||||||||||||||||
| 7 Period 7 element A period 7 element is one of the chemical elements in the seventh row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical... |
||||||||||||||||||||
| *Lanthanoids | ||||||||||||||||||||
| **Actinoids | ||||||||||||||||||||
- Green box = Carbene complex with element known.
- Grey box = No carbene complex with element known.
Figure: Periodic Table featuring elements that have formed stable carbenes complexes.
Stable carbenes are believed to behave in a similar fashion to organophosphines in their co-ordination properties to metals. These ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s are said to be good σ-donors through the carbenic lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
, but poor π-acceptors due to internal ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
back-donation from the nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
atoms adjacent to the carbene centre, and so are able to co-ordinate to even relatively electron deficient metals. Enders and Hermann have shown that these carbenes are suitable replacements for phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
ligands in several catalytic cycles. Whilst they have found that these ligands do not activate the metal catalyst as much as phosphine ligands they often result in more robust catalysts. Several catalytic systems have been looked into by Hermann and Enders, using catalysts containing imidazole and triazole carbene ligands, with moderate success. Grubbs has reported replacing a phosphine ligand (PCy3) with an imidazol-2-ylidene in the olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
catalyst RuCl2(PCy3)2CHPh, and noted increased ring closing metathesis as well as exhibiting “a remarkable air and water stability”. Molecules containing two and three carbene moieties have been prepared as potential bidentate and tridentate carbene ligands.
Carbenes in organometallic chemistry & catalysis
Carbenes can be stabilised as organometallicOrganometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
species. These transition metal carbene complex
Transition metal carbene complex
A transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and...
es fall into two categories:
- FischerErnst Otto FischerErnst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.-Early life:...
carbenes in which carbenes are tethered to a metal and an electron-withdrawing group (usually a carbonyl), - SchrockRichard R. SchrockRichard Royce Schrock is an American chemist and Nobel laureate recognized for his contributions to the metathesis reaction used in organic chemistry.-Biography:...
carbenes; in which carbenes are tethered to a metal and an electron-donating group. The reactions that such carbenes participate in are very different from those in which organic carbenes participate.
Triplet state carbene chemistry
Persistent triplet state carbenes are likely to have very similar reactivity as other non-persistent triplet state carbenes.Physical properties
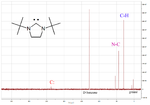
One of the more useful physical properties is the diagnostic chemical shift of the carbenic carbon atom in the 13C-NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
spectrum. Typically this peak is in the range between 200 and 300 ppm, where few other peaks appear in the 13C-NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
spectrum. An example is shown on the left for a cyclic diaminocarbene which has a carbenic peak at 238 ppm.
Upon coordination to metal centers, the 13C carbene resonance usually shifts highfield, depending on the Lewis acidity of the complex fragment. Based on this observation, Huynh et al. developed a new methodology to determine ligand donor strengths by 13C NMR analysis of trans-palladium(II)-carbene complexes. The use of a 13C-labeled N-heterocyclic carbene ligand also allows for the study of mixed carbene-phosphine complexes, which undergo trans-cis-isomerization due to the transphobia effect.
Applications
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in organometallic chemistry. Recently there have been practical application of these carbenes as metal ligands in catalysis, e.g. in the ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
-based Grubbs' catalyst
Grubbs' catalyst
Grubbs' Catalyst is a transition metal carbene complex named after Robert H. Grubbs, the chemist who first synthesized it. There are two generations of the catalyst, as shown on the right. In contrast to other olefin metathesis catalysts, Grubbs' Catalysts tolerate other functional groups in the...
and palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
-based catalysts for cross-coupling reactions.
Preparation methods
Stable carbenes are very reactive molecules and so it is important to consider the reaction conditions carefully when attempting to prepare these molecules. Stable carbenes are strongly basicBase (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
(the pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
value of the conjugate acid
Conjugate acid
Within the Brønsted–Lowry acid-base theory , a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases, or donates, a proton in the forward chemical...
of an imidazol-2-ylidene was measured at ca. 24) and react with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. Clearly these reactions must be performed under a dry, inert atmosphere, avoiding protic solvents or compounds of even moderate acidity. Furthermore, one must also consider the relative stability of the starting materials. Whilst imidazolium salts are stable to nucleophilic addition, other non-aromatic salts are not (i.e. formamidinium
Formamidinium
Formamidinium cations are positively charged organic chemical compounds which can be represented by the chemical formula [R2N-CH=NR2]+.-Chemical applications:...
salts).
Consequently in these cases, strong unhindered nucleophiles must be avoided whether they are generated in situ or are present as an impurity in other reagents (e.g. LiOH in BuLi).
Several approaches have been developed in order to prepare stable carbenes, these are outlined below.
Deprotonation
DeprotonationDeprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of carbene precursor salts with strong bases has proved a reliable route to almost all stable carbenes:
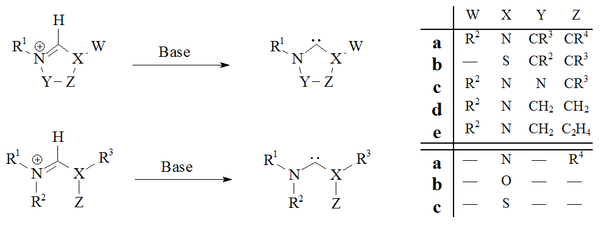
Several bases and reaction conditions have been employed with varying success. The degree of success has been principally dependent on the nature of the precursor being deprotonated. The major drawback with this method of preparation is the problem of isolation of the free carbene from the metals ions used in their preparation.
Metal hydride bases
One might believe that sodium or potassium hydridePotassium hydride
Potassium hydride, KH, is a chemical compound of potassium and hydrogen. It is a hydride of potassium. It reacts with water according to the reaction:...
would be the ideal base for deprotonating these precursor salts. The hydride should react irreversibly with the loss of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
to give the desired carbene, with the inorganic by-products and excess hydride being removed by filtration. In practice this reaction is often too slow in suitable solvents (e.g. THF) due to the relative insolubility of the metal hydride and the salt.
The addition of soluble "catalysts" (DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, t-BuOH
Tert-Butanol
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether...
) considerably improves the rate of reaction of this heterogeneous system, via the generation of tert-butoxide or dimsyl anion. However, these catalysts have proved ineffective for the preparation of non-imidazolium adducts as they tend to act as nucleophiles towards the precursor salts and in so doing are destroyed. The presence of hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ions as an impurity in the metal hydride could also destroy non-aromatic salts.
Deprotonation with sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
or potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
hydride in a mixture of liquid ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
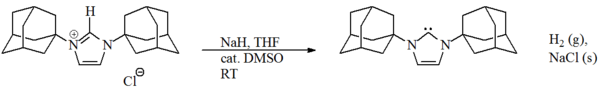
/THF at -40 °C has been reported to work well by Hermann and others for imidazole based carbenes. Arduengo and co-workers managed to prepare a dihydroimidazol-2-ylidene using NaH. However, this method has not been applied to the preparation of diaminocarbenes.
Potassium tert-butoxide
Arduengo and co-workers have used potassium tert-butoxidePotassium tert-butoxide
Potassium tert-butoxide is the chemical compound with the formula 3COK. This colourless solid is a strong base useful in organic synthesis. It exists as a tetrameric cubane-like cluster...
without the addition of a metal hydride to deprotonate precursor salts.
Alkyllithiums
The use of alkyllithiums as strong bases has not been extensively studied, and have been unreliable for deprotonation of precursor salts. With non-aromatic salts, n-BuLi and PhLi can act as nucleophiles whilst t-BuLi can on occasion act as a source of hydride, reducing the salt with the generation of isobutene: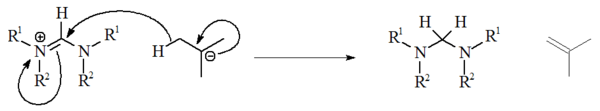
Lithium amides
Lithium amides like the diisopropylamide (LDA)Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
and the (tetramethylpiperidide (LiTMP)
Lithium tetramethylpiperidide
Lithium tetramethylpiperidide or LiTMP is an organic base and a non-nucleophilic base. It is traditionally synthesised from 2,2,6,6-tetramethylpiperidine and n-butyllithium at -78 °C, but recent reports show this reaction is sluggish and is better formed at 0 °C. The compound is stable in a...
) generally work well for the deprotonation of all types of salts, providing that not too much LiOH
Lithium hydroxide
Lithium hydroxide is an inorganic compound with the formula LiOH. It is a white hygroscopic crystalline material. It is soluble in water and slightly soluble in ethanol...
is present in the n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
used to make the lithium amide. Titration of lithium amide can be used to determine the amount of hydroxide in solution.
Metal hexamethyldisilazides
The deprotonation of precursor salts with metal hexamethyldisilazides works very cleanly for the deprotonation of all types of salts, except for unhindered formamidinium salts, where this base can act as a nucleophile to give a triaminomethane adduct.Metal free carbene preparation
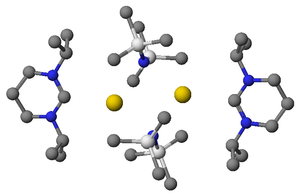
Shown right is an X-ray structure showing a complex between a diaminocarbene and potassium HMDS
Bis(trimethylsilyl)amine
Bisamine is an organosilicon compound with the molecular formula [3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms...
. This complex was formed when excess KHMDS
Potassium bis(trimethylsilyl)amide
Potassium bisamide is the chemical compound with the formula 2NK...
was used as a strong base to deprotonate the formamidinium
Formamidinium
Formamidinium cations are positively charged organic chemical compounds which can be represented by the chemical formula [R2N-CH=NR2]+.-Chemical applications:...
salt. Removing lithium ions resulting from deprotonation with reagents such as LDA
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
can be especially problematic. Potassium and sodium salt by-products tend to precipitate from solution and can be removed. Lithium ions may be chemically removed by binding to species such as cryptand
Cryptand
Cryptands are a family of synthetic bi- and polycyclic multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their efforts in discovering and determining uses of cryptands and crown ethers,...
s or crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
s.
Metal free carbenes have been prepared in several ways as outlined below:
Dechalcogenation
Another approach of preparing carbenes has relied on the desulfurisationDesulfurisation
Desulfurisation is a chemical reaction involving the removal of sulfur from a molecule .An important industrial desulfurisation processes is also the hydro-desulfurization of natural gas...
of thiourea
Thiourea
Thiourea is an organosulfur compound of with the formula SC2 . It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad...
s with molten potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
in boiling THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
. A contributing factor to the success of this reaction is that the byproduct, potassium sulfide
Potassium sulfide
Potassium sulfide is the inorganic compound with the formula K2S. The colourless solid is rarely encountered, because it reacts readily with water, a reaction that affords potassium bisulfide and potassium hydroxide .-Structure:...
, is insoluble in the solvent. The elevated temperatures suggest that this method is not suitable for the preparation of unstable dimerising carbenes. A single example of the deoxygenation
Deoxygenation
Deoxygenation is a chemical reaction involving the removal of molecular oxygen from a reaction mixture or solvent, or the removal of oxygen atoms from a molecule.Classic representatives of deoxygenation are:...
of a urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
with a fluorene
Fluorene
Fluorene, or 9H-fluorene, is a polycyclic aromatic hydrocarbon. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It is combustible. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar...
derived carbene to give the tetramethyldiaminocarbene and fluorenone has also been reported:

Desulfurisation
Desulfurisation is a chemical reaction involving the removal of sulfur from a molecule .An important industrial desulfurisation processes is also the hydro-desulfurization of natural gas...
of thiourea
Thiourea
Thiourea is an organosulfur compound of with the formula SC2 . It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad...
s with molten potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
to give imidazol-2-ylidenes or diaminocarbenes has not been widely used. The method was used to prepare dihydroimidazole carbenes.
Vacuum pyrolysis
Vacuum pyrolysis, with the removal of neutral volatile by-products (CH3OH, CHCl3), has been used to prepare dihydroimidazole and triazole based carbenes:
Bis(trimethylsilyl)mercury
Bis(trimethylsilyl)mercuryBis(trimethylsilyl)mercury
Bismercury a chemical reagent with formula: 3-Si-Hg-Si-3.- Synthesis :This compound was first synthesized by Wiberg et al. in 1963, by the reaction of trimethylsilyl bromide with sodium amalgam:- Reactions :...
(CH3)3Si-Hg-Si(CH3)3 reacts with chloro-iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
and chloro-amidinium
Amidinium
Amidinium cations are positively charged organic chemical compounds which can be represented by the chemical formula H2N=CH-NH2+. Substituted version are known as formamidinium salts....
salts to give a metal-free carbene and elemental mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
. For example, (CH3)3Si-Hg-Si(CH3)3 + R2N=C(Cl)-NR2+ Cl- → R2N-C:-NR2 + Hg(l) + (CH3)3Si-Cl
Photochemical decomposition
Persistent triplet state carbenes have been prepared by photochemical decomposition of a diazomethaneDiazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
product via the expulsion of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
gas, at a wavelength of 300 nm in benzene.
Purification
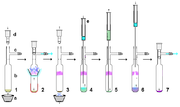
Air-free technique
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen...
s. However, provided rigorously dry, relatively non-acidic and air-free materials are used, stable carbenes are reasonably robust to handling per se. By way of example, a stable carbene prepared from potassium hydride can be filtered through a dry celite pad to remove excess KH (and resulting salts) from the reaction. On a relatively small scale, a suspension containing a stable carbene in solution can be allowed to settle and the supernatant solution pushed through a dried membrane syringe filter. Stable carbenes are readily soluble in non-polar solvents such as hexane, and so typically recrystallisation
Recrystallization (chemistry)
-Chemistry:In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted , which includes recrystallization...
of stable carbenes can be difficult, due to the unavailability of suitable non-acidic polar solvents. Air-free sublimation as shown right can be an effective method of purification, although temperatures below 60 °C under high vacuum are preferable as these carbenes are relatively volatile and also could begin to decompose at these higher temperatures. Indeed, sublimation in some cases can give single crystals suitable for X-ray analysis. However, strong complexation to metal ions like lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
will in most cases prevent sublimation.
Further reading
Reviews on persistent carbenes:- Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents, (Chapter 4, Hideo Tomioka (triplet state); Chapter 5 (singlet state), Roger W. Alder) - ed. Guy Bertrand
- Reactive Intermediate Chemistry By Robert A. Moss, Matthew Platz, Maitland Jones (Chapter 8, Stable Singlet Carbenes, Guy Bertrand)
- R. W. Alder, in 'Diaminocarbenes: exploring structure and reactivity', ed. G. Bertrand, New York, 2002
For a review on the physico-chemical properties (electronics, sterics, ...) of N-heterocyclic carbenes: [Angew. Chem. 2010, 122, 7094-7107.]

