Pentacene
Encyclopedia
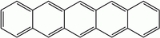
Pentacene is a polycyclic aromatic hydrocarbon
consisting of five linearly-fused benzene
rings. This highly conjugated compound is an organic semiconductor
. The compound generates exciton
s upon absorption of ultra-violet (UV) or visible light
; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.
Structurally, pentacene is one of the linear acene
s, the previous one being tetracene
(four fused benzene rings) and the next one being hexacene
(six fused benzene rings). In August 2009, a group of researchers from IBM published results experimental results of imaging a single molecule
of pentacene using an atomic force microscope
. In July 2011, they used a modification of scanning tunneling microscopy to experimentally determine the shapes of the highest occupied and lowest unoccupied molecular orbitals
.
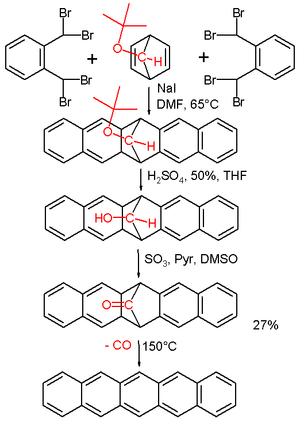
 A classic method for pentacene synthesis is by the Elbs reaction
A classic method for pentacene synthesis is by the Elbs reaction
. Pentacenes can also be prepared in the laboratory by extrusion of a small volatile component. In one such experimental procedure carbon monoxide
is liberated from a precursor at 150 °C. The precursor is reported to have some solubility in chloroform
and is therefore amiable to spin coating
. Pentacene is soluble in hot chlorinated benzenes, such as 1,2,4-trichlorobenzene
, from which it can be recrystallized to form platelets.
Although pentacene's structure resembles that of other aromatic compounds like anthracene
, its aromatic properties are poorly defined; as such, pentacene and its derivatives are the subject of much research.
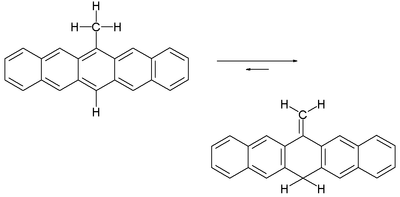
A tautomer
ic chemical equilibrium
exists between 6-methylene-6,13-dihydropentacene and 6-methylpentacene.
This equilibrium is entirely in favor of the methylene compound. Only by heating a solution of the compound to 200 °C does a small amount of the pentacene develop, as evidenced by the emergence of a red-violet color. According to one study the reaction mechanism
for this equilibrium is not based on an intramolecular
1,5-hydride shift, but on a bimolecular free radical hydrogen migration. In contrast, isotoluene
s with the same central chemical motif easily aromatize.
Pentacene reacts with elemental sulfur
in 1,2,4-trichlorobenzene
to the compound hexathiapentacene. X-ray crystallography
shows that all the carbon-to-sulfur bond length
s are roughly equal (170 pm); from this, it follows that resonance structures B and C with complete charge separation are more significant than structure A.
In the crystal phase the molecules display aromatic stacking interactions, whereby the distance between some sulfur atoms on neighboring molecules can become less (337 pm) than the sum of two Van der Waals radii
(180 pm)
Like the related tetrathiafulvalene
, this compound is studied in the field of organic semiconductor
s.
The acenes may appear as planar and rigid molecules, but in fact they can be very distorted. The pentacene depicted below:
has an end-to end twist of 144° and is sterically stabilized by the six phenyl groups. The compound can be resolved into its two enantiomer
s with an unusually high reported optical rotation
of 7400° although racemization
takes place with a chemical half-life of 9 hours.
Oligomers and polymers based on pentacene have been explored both synthetically as well as in device application settings. Polymer light emitting diodes (PLEDs) have been constructed using conjugated copolymers (1a–b) containing fluorene and pentacene. A few other conjugated pentacene polymers (2a–b and 3) have been realized based on Sonogashira
and Suzuki
coupling reactions of a dibromopentacene monomer. Non-conjugated pentacene-based polymers have been synthesized via esterification of a pentacene diol monomer with bis-acid chlorides to from polymers 4a–b.
Various synthetic strategies have been employed to form conjugated oligomers of pentacene 5a–c including a one-pot-four-bond forming procedure which provided a solution-processable conjugated pentacene dimer (5c) which exhibited photoconductive gain >10, placing its performance within the same order of magnitude as thermally evaporated films of non-functionalized pentacene which exhibited photoconductive gain >16 using analogous measurement techniques. A modular synthetic method to conjugated pentacene di-, tri- and tetramers (6–8) has been reported which is based on homo- and cross-coupling reactions of robust dehydropentacene intermediates. Non-conjugated oligomers 9–10 based on pentacene have been synthesized, including dendrimers 9–10 with up to 9 pentacene moieties per molecule with molar absorptivity for the most intense absorption > 2,000,000 M−1•cm−1. Dendrimers 11–12 were shown to have improved performance in devices compared to analogous pentacene-based polymers 4a–b in the context of photodetectors.
phase.
Combined with buckminsterfullerene
, pentacene is used in the development of organic photovoltaic prototypes.
Pentacene is a popular choice for research on organic thin film transistors and OFET
s, being one of the most thoroughly investigated conjugated organic molecules with a high application potential due to a hole mobility in OFETs of up to 5.5 cm2/(V·s), which exceeds that of amorphous silicon.
Pentacene, as well as other organic conductors, is subject rapid oxidation in air, which precludes commercialization. If the pentacene preoxidized, the pentacene-quinone is a potential gate insulator, then the mobility can approach that of rubrene
– the highest-mobility organic semiconductor – namely, 40 cm2/(V·s). This pentacene oxidation technique is akin to the silicon oxidation used in the silicon electronics.
Polycyclic aromatic hydrocarbon
Polycyclic aromatic hydrocarbons , also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons, are potent atmospheric pollutants that consist of fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH...
consisting of five linearly-fused benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
rings. This highly conjugated compound is an organic semiconductor
Organic semiconductor
An organic semiconductor is an organic material with semiconductor properties. Single molecules, short chain and organic polymers can be semiconductive. Semiconducting small molecules include the polycyclic aromatic compounds pentacene, anthracene, and rubrene...
. The compound generates exciton
Exciton
An exciton is a bound state of an electron and hole which are attracted to each other by the electrostatic Coulomb force. It is an electrically neutral quasiparticle that exists in insulators, semiconductors and some liquids...
s upon absorption of ultra-violet (UV) or visible light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.
Structurally, pentacene is one of the linear acene
Acene
Acenes or polyacenes is a class of organic compounds and polycyclic aromatic hydrocarbons made up of linearly fused benzene rings. The larger representatives have potential interest in optoelectronic applications and are actively researched in chemistry and electrical engineering...
s, the previous one being tetracene
Tetracene
Tetracene, also called naphthacene, is a polycyclic aromatic hydrocarbon. It has the appearance of a pale orange powder. Tetracene is the four-ringed member of the series of acenes, the previous one being anthracene and the next one being pentacene.Tetracene is a molecular organic semiconductor,...
(four fused benzene rings) and the next one being hexacene
Hexacene
Hexacene is an aromatic molecule consisting of six linearly-fused benzene rings. Hexacene and its derivatives are investigated for potential applications as organic semiconductor....
(six fused benzene rings). In August 2009, a group of researchers from IBM published results experimental results of imaging a single molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
of pentacene using an atomic force microscope
Atomic force microscope
Atomic force microscopy or scanning force microscopy is a very high-resolution type of scanning probe microscopy, with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit...
. In July 2011, they used a modification of scanning tunneling microscopy to experimentally determine the shapes of the highest occupied and lowest unoccupied molecular orbitals
HOMO/LUMO
HOMO and LUMO are acronyms for highest occupied molecular orbital and lowest unoccupied molecular orbital, respectively. The energy difference between the HOMO and LUMO is termed the HOMO-LUMO gap...
.
Synthesis

Elbs reaction
The Elbs reaction is an organic reaction describing the pyrolysis of an ortho methyl substituted benzophenone to condensed polyaromatic. The reaction is named after its inventor, the German chemist Karl Elbs also responsible for the Elbs oxidation...
. Pentacenes can also be prepared in the laboratory by extrusion of a small volatile component. In one such experimental procedure carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
is liberated from a precursor at 150 °C. The precursor is reported to have some solubility in chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
and is therefore amiable to spin coating
Spin coating
Spin coating is a procedure used to apply uniform thin films to flat substrates. In short, an excess amount of a solution is placed on the substrate, which is then rotated at high speed in order to spread the fluid by centrifugal force...
. Pentacene is soluble in hot chlorinated benzenes, such as 1,2,4-trichlorobenzene
1,2,4-Trichlorobenzene
1,2,4-Trichlorobenzene is an organic compound used as a solvent, and is one of the best known solvents used to dissolve fullerenes and pentacene. It is a benzene derivative with three chlorine atoms substitutents, in the 1, 2 and 4 positions of the benzene ring.-See...
, from which it can be recrystallized to form platelets.
Monomeric pentacene derivatives
6,13-Substituted pentacenes are accessible through pentacenequinone by reaction with an aryl or alkynyl nucleophile (for example Grignard or organolithium reagents) followed by reductive aromatization. Another method is based on homologization of diynes by transition metals (through zirconacyclopentadienes) Functionalization of pentacene has allowed for control of the solid-state packing of this chromophore. The choice of the substituents (both size and location of substitution on the pentacene) influences the solid-state packing and can be used to control whether the compound adopts 1-dimensional or 2-dimensional cofacial pi-stacking in the solid-state, as opposed to the herringbone packing observed for pentacene.Although pentacene's structure resembles that of other aromatic compounds like anthracene
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon consisting of three fused benzene rings. It is a component of coal-tar. Anthracene is used in the production of the red dye alizarin and other dyes...
, its aromatic properties are poorly defined; as such, pentacene and its derivatives are the subject of much research.
A tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
ic chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
exists between 6-methylene-6,13-dihydropentacene and 6-methylpentacene.
This equilibrium is entirely in favor of the methylene compound. Only by heating a solution of the compound to 200 °C does a small amount of the pentacene develop, as evidenced by the emergence of a red-violet color. According to one study the reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for this equilibrium is not based on an intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
1,5-hydride shift, but on a bimolecular free radical hydrogen migration. In contrast, isotoluene
Isotoluene
The isotoluenes in organic chemistry are the non-aromatic toluene isomers with an exocyclic double bond. They are of some academic interest in relation to aromaticity and isomerisation mechanisms....
s with the same central chemical motif easily aromatize.
Pentacene reacts with elemental sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
in 1,2,4-trichlorobenzene
Trichlorobenzene
Trichlorobenzene may refer to any of three isomeric chlorinated derivatives of benzene with the molecular formula C6H3Cl3:* 1,2,3-Trichlorobenzene* 1,2,4-Trichlorobenzene* 1,3,5-Trichlorobenzene...
to the compound hexathiapentacene. X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
shows that all the carbon-to-sulfur bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s are roughly equal (170 pm); from this, it follows that resonance structures B and C with complete charge separation are more significant than structure A.
In the crystal phase the molecules display aromatic stacking interactions, whereby the distance between some sulfur atoms on neighboring molecules can become less (337 pm) than the sum of two Van der Waals radii
Van der Waals radius
The van der Waals radius, r, of an atom is the radius of an imaginary hard sphere which can be used to model the atom for many purposes. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms had a finite size and to...
(180 pm)
Like the related tetrathiafulvalene
Tetrathiafulvalene
Tetrathiafulvalene is a organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, 2, by replacement of four CH groups with sulfur atoms...
, this compound is studied in the field of organic semiconductor
Organic semiconductor
An organic semiconductor is an organic material with semiconductor properties. Single molecules, short chain and organic polymers can be semiconductive. Semiconducting small molecules include the polycyclic aromatic compounds pentacene, anthracene, and rubrene...
s.
The acenes may appear as planar and rigid molecules, but in fact they can be very distorted. The pentacene depicted below:
has an end-to end twist of 144° and is sterically stabilized by the six phenyl groups. The compound can be resolved into its two enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s with an unusually high reported optical rotation
Optical rotation
Optical rotation is the turning of the plane of linearly polarized light about the direction of motion as the light travels through certain materials. It occurs in solutions of chiral molecules such as sucrose , solids with rotated crystal planes such as quartz, and spin-polarized gases of atoms...
of 7400° although racemization
Racemization
In chemistry, racemization refers to the converting of an enantiomerically pure mixture into a mixture where more than one of the enantiomers are present...
takes place with a chemical half-life of 9 hours.
Oligomers and polymers of pentacene
Oligomers and polymers based on pentacene have been explored both synthetically as well as in device application settings. Polymer light emitting diodes (PLEDs) have been constructed using conjugated copolymers (1a–b) containing fluorene and pentacene. A few other conjugated pentacene polymers (2a–b and 3) have been realized based on Sonogashira
Sonogashira coupling
In organic chemistry, a Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides. This reaction was first reported by Kenkichi Sonogashira and Nobue Hagihara in 1975.-Catalyst:...
and Suzuki
Suzuki
is a Japanese multinational corporation headquartered in Hamamatsu, Japan that specializes in manufacturing compact automobiles and 4x4 vehicles, a full range of motorcycles, all-terrain vehicles , outboard marine engines, wheelchairs and a variety of other small internal combustion engines...
coupling reactions of a dibromopentacene monomer. Non-conjugated pentacene-based polymers have been synthesized via esterification of a pentacene diol monomer with bis-acid chlorides to from polymers 4a–b.
Various synthetic strategies have been employed to form conjugated oligomers of pentacene 5a–c including a one-pot-four-bond forming procedure which provided a solution-processable conjugated pentacene dimer (5c) which exhibited photoconductive gain >10, placing its performance within the same order of magnitude as thermally evaporated films of non-functionalized pentacene which exhibited photoconductive gain >16 using analogous measurement techniques. A modular synthetic method to conjugated pentacene di-, tri- and tetramers (6–8) has been reported which is based on homo- and cross-coupling reactions of robust dehydropentacene intermediates. Non-conjugated oligomers 9–10 based on pentacene have been synthesized, including dendrimers 9–10 with up to 9 pentacene moieties per molecule with molar absorptivity for the most intense absorption > 2,000,000 M−1•cm−1. Dendrimers 11–12 were shown to have improved performance in devices compared to analogous pentacene-based polymers 4a–b in the context of photodetectors.
Materials research
Pentacenes have been examined as potential dichroic dyes. The pentacenoquinone displayed below is fluorescent and when mixed with liquid crystal E7 mixture a dichroic ratio of 8 is reached. Longer acene align better in the nematic liquid crystalLiquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
phase.
Combined with buckminsterfullerene
Buckminsterfullerene
Buckminsterfullerene is a spherical fullerene molecule with the formula . It was first intentionally prepared in 1985 by Harold Kroto, James Heath, Sean O'Brien, Robert Curl and Richard Smalley at Rice University...
, pentacene is used in the development of organic photovoltaic prototypes.
Pentacene is a popular choice for research on organic thin film transistors and OFET
OFET
An organic field-effect transistor is a field effect transistor using an organic semiconductor in its channel. OFETs can be prepared either by vacuum evaporation of small molecules, by solution-casting of polymers or small molecules, or by mechanical transfer of a peeled single-crystalline organic...
s, being one of the most thoroughly investigated conjugated organic molecules with a high application potential due to a hole mobility in OFETs of up to 5.5 cm2/(V·s), which exceeds that of amorphous silicon.
Pentacene, as well as other organic conductors, is subject rapid oxidation in air, which precludes commercialization. If the pentacene preoxidized, the pentacene-quinone is a potential gate insulator, then the mobility can approach that of rubrene
Rubrene
Rubrene is a red colored polycyclic aromatic hydrocarbon. Rubrene is used as a sensitiser in chemoluminescence and as a yellow light source in lightsticks....
– the highest-mobility organic semiconductor – namely, 40 cm2/(V·s). This pentacene oxidation technique is akin to the silicon oxidation used in the silicon electronics.
External links
- facts about pentacene, retrieved Apr. 17, 2006
- Organic transistor improves with age, New Scientist, 2 December 2007
- Pentacene Imaged, IBM images Pentacene, the first molecule imaged in detail 29 August 2009