
Penning ionization
Encyclopedia
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms and/or molecules. The process is named after the Dutch physicist Frans Michel Penning
who first reported it in 1927.
The Penning effect is put to practical use in applications such as gas-discharge neon lamp
s and fluorescent lamp
s, where the lamp is filled with a Penning mixture
to improve the electrical characteristics of the lamps.
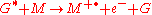
Penning ionization occurs when the target molecule has an ionization potential
lower than the internal energy of the excited-state atom or molecule. Associative Penning ionization can also occur:
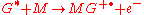
Surface Penning ionization refers to the interaction of the excited-state gas with a surface S, resulting in the release of an electron.

(The positive charge symbol that would appear to be required for charge conservation is omitted, because S is a macroscopic surface and the loss of one electron has a negligible effect.)
that would appear to be required for charge conservation is omitted, because S is a macroscopic surface and the loss of one electron has a negligible effect.)
Frans Michel Penning
Frans Michel Penning was a Dutch physicist. The Penning trap, used for storing charged particles, as well as the Penning mixture and Penning effect of gas discharge tubes are named after him. He also invented a type of cold cathode vacuum gauge known as Penning gauge.-External links:*...
who first reported it in 1927.
The Penning effect is put to practical use in applications such as gas-discharge neon lamp
Neon lamp
A neon lamp is a miniature gas discharge lamp that typically contains neon gas at a low pressure in a glass capsule. Only a thin region adjacent to the electrodes glows in these lamps, which distinguishes them from the much longer and brighter neon tubes used for signage...
s and fluorescent lamp
Fluorescent lamp
A fluorescent lamp or fluorescent tube is a gas-discharge lamp that uses electricity to excite mercury vapor. The excited mercury atoms produce short-wave ultraviolet light that then causes a phosphor to fluoresce, producing visible light. A fluorescent lamp converts electrical power into useful...
s, where the lamp is filled with a Penning mixture
Penning mixture
A Penning mixture , named after Frans Michel Penning, is a mixture of gases used in electric lighting or displaying fixtures. Although the popular phrase for the most common of these is a neon lamp, it's more efficient to have the glass tube filled not with pure neon, but with a Penning mixture,...
to improve the electrical characteristics of the lamps.
Reactions
Penning ionization refers to the interaction between a gas-phase excited-state atom or molecule G* and a target molecule M resulting in the formation of a radical molecular cation M+., an electron e−, and a neutral gas molecule G: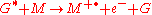
Penning ionization occurs when the target molecule has an ionization potential
Ionization potential
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
lower than the internal energy of the excited-state atom or molecule. Associative Penning ionization can also occur:
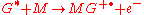
Surface Penning ionization refers to the interaction of the excited-state gas with a surface S, resulting in the release of an electron.

(The positive charge symbol
 that would appear to be required for charge conservation is omitted, because S is a macroscopic surface and the loss of one electron has a negligible effect.)
that would appear to be required for charge conservation is omitted, because S is a macroscopic surface and the loss of one electron has a negligible effect.)

