
Partial molar property
Encyclopedia
A partial molar property is a thermodynamic
quantity which indicates how an extensive property
of a solution
or mixture
varies with changes in the molar
composition of the mixture at constant temperature
and pressure
, or for constant values of the natural variables of the extensive property considered. Essentially it is the partial derivative with respect to the quantity (number of moles) of the component of interest. Every extensive property of a mixture has a corresponding partial molar property.
is broadly understood as the contribution that a component of a mixture makes to the overall volume of the solution. However, there is rather more to it than this:
When one mole of water is added to a large volume of water at 25 ºC, the volume increases by 18 cm3. The molar volume of pure water would thus be reported as 18 cm3 mol-1. However, addition of one mole of water to a large volume of pure ethanol
results in an increase in volume of only 14 cm3. The reason that the increase is different is that the volume occupied by a given number of water molecules depends upon the identity of the surrounding molecules. The value 14 cm3 is said to be the partial molar volume of water in ethanol.
In general, the partial molar volume of a substance X in a mixture is the change in volume per mole of X added to the mixture.
The partial molar volumes of the components of a mixture vary with the composition of the mixture, because the environment of the molecules in the mixture changes with the composition. It is the changing molecular environment (and the consequent alteration of the interactions between molecules) that results in the thermodynamic properties of a mixture changing as its composition is altered
If, by , one denotes a generic extensive property of a mixture, it will always be true that it depends on the pressure
, one denotes a generic extensive property of a mixture, it will always be true that it depends on the pressure
(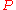 ), temperature
), temperature
( ), and the amount of each component of the mixture (measured in moles
), and the amount of each component of the mixture (measured in moles
, n). For a mixture with m components, this is expressed as
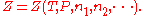
Now if temperature T and pressure P are held constant,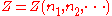 is a homogeneous function
is a homogeneous function
of degree 1, since doubling the quantities of each component in the mixture will double . More generally, for any
. More generally, for any 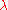 :
:

By Euler's first theorem for homogeneous functions, this implies
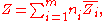
where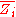 is the partial molar
is the partial molar  of component
of component 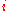 defined as:
defined as:
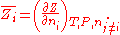
By Euler's second theorem for homogeneous functions,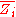 is a homogeneous function of degree 0 which means that for any
is a homogeneous function of degree 0 which means that for any 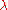 :
:
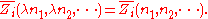
In particular, taking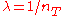 where
where  , one has
, one has
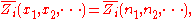
where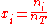 is the concentration
is the concentration
, or mole fraction of component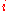 .
.
Since the molar fractions satisfy the relation
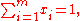
the xi are not independent, and the partial molar property is a function of only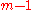 mole fractions:
mole fractions:
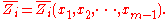
The partial molar property is thus an intensive property
- it does not depend on the size of the system.
s are often maintained at constant temperature and pressure and under these conditions, the value of any extensive property
can be obtained from its partial molar property. They are especially useful when considering specific properties of pure substances (that is, properties of one mole of pure substance) and properties of mixing. By definition, properties of mixing are related to those of the pure substance by:
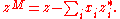
Here denotes the pure substance,
denotes the pure substance,  the mixing property, and
the mixing property, and  corresponds to the specific property under consideration. From the definition of partial molar properties,
corresponds to the specific property under consideration. From the definition of partial molar properties,
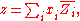
substitution yields:
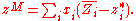
So from knowledge of the partial molar properties, properties of mixing can be calculated.
U, enthalpy
H, Helmholtz free energy
A, and Gibbs free energy
G, the following hold:
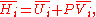
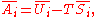
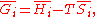
where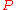 is the pressure,
is the pressure,  the volume
the volume
, the temperature, and
the temperature, and 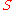 the entropy
the entropy
.
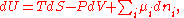
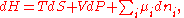
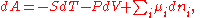
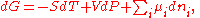
where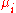 is the chemical potential
is the chemical potential
defined as (for constant nj with j≠i):

This last partial derivative
is the same as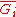 , the partial molar Gibbs free energy
, the partial molar Gibbs free energy
. This means that the partial molar Gibbs free energy and the chemical potential, one of the most important properties in thermodynamics and chemistry, are the same quantity. Under isobaric
(constant P) and isothermal
(constant T ) conditions, knowledge of the chemical potentials,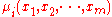 , yields every property of the mixture as they completely determine the Gibbs free energy.
, yields every property of the mixture as they completely determine the Gibbs free energy.
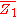 of a binary solution, one begins with the pure component denoted as
of a binary solution, one begins with the pure component denoted as 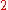 and, keeping the temperature and pressure constant during the entire process, add small quantities of component
and, keeping the temperature and pressure constant during the entire process, add small quantities of component 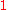 ; measuring
; measuring  after each addition. After sampling the compositions of interest one can fit a curve to the experimental data. This function will be
after each addition. After sampling the compositions of interest one can fit a curve to the experimental data. This function will be 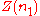 .
.
Differentiating with respect to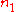 will give
will give 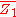 .
.
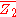 is then obtained from the relation:
is then obtained from the relation:
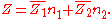
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
quantity which indicates how an extensive property
Intensive and extensive properties
In the physical sciences, an intensive property , is a physical property of a system that does not depend on the system size or the amount of material in the system: it is scale invariant.By contrast, an extensive property In the physical sciences, an intensive property (also called a bulk...
of a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
or mixture
Mixture
In chemistry, a mixture is a material system made up by two or more different substances which are mixed together but are not combined chemically...
varies with changes in the molar
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
composition of the mixture at constant temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, or for constant values of the natural variables of the extensive property considered. Essentially it is the partial derivative with respect to the quantity (number of moles) of the component of interest. Every extensive property of a mixture has a corresponding partial molar property.
Definition
The partial molar volumeMolar volume
The molar volume, symbol Vm, is the volume occupied by one mole of a substance at a given temperature and pressure. It is equal to the molar mass divided by the mass density...
is broadly understood as the contribution that a component of a mixture makes to the overall volume of the solution. However, there is rather more to it than this:
When one mole of water is added to a large volume of water at 25 ºC, the volume increases by 18 cm3. The molar volume of pure water would thus be reported as 18 cm3 mol-1. However, addition of one mole of water to a large volume of pure ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
results in an increase in volume of only 14 cm3. The reason that the increase is different is that the volume occupied by a given number of water molecules depends upon the identity of the surrounding molecules. The value 14 cm3 is said to be the partial molar volume of water in ethanol.
In general, the partial molar volume of a substance X in a mixture is the change in volume per mole of X added to the mixture.
The partial molar volumes of the components of a mixture vary with the composition of the mixture, because the environment of the molecules in the mixture changes with the composition. It is the changing molecular environment (and the consequent alteration of the interactions between molecules) that results in the thermodynamic properties of a mixture changing as its composition is altered
If, by
 , one denotes a generic extensive property of a mixture, it will always be true that it depends on the pressure
, one denotes a generic extensive property of a mixture, it will always be true that it depends on the pressurePressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
(
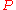 ), temperature
), temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
(
 ), and the amount of each component of the mixture (measured in moles
), and the amount of each component of the mixture (measured in molesMole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
, n). For a mixture with m components, this is expressed as
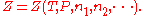
Now if temperature T and pressure P are held constant,
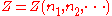 is a homogeneous function
is a homogeneous functionHomogeneous function
In mathematics, a homogeneous function is a function with multiplicative scaling behaviour: if the argument is multiplied by a factor, then the result is multiplied by some power of this factor. More precisely, if is a function between two vector spaces over a field F, and k is an integer, then...
of degree 1, since doubling the quantities of each component in the mixture will double
 . More generally, for any
. More generally, for any 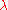 :
:
By Euler's first theorem for homogeneous functions, this implies
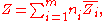
where
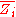 is the partial molar
is the partial molar  of component
of component 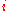 defined as:
defined as: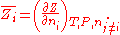
By Euler's second theorem for homogeneous functions,
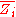 is a homogeneous function of degree 0 which means that for any
is a homogeneous function of degree 0 which means that for any 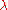 :
: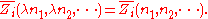
In particular, taking
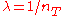 where
where  , one has
, one has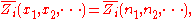
where
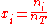 is the concentration
is the concentrationConcentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
, or mole fraction of component
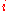 .
.Since the molar fractions satisfy the relation
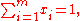
the xi are not independent, and the partial molar property is a function of only
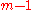 mole fractions:
mole fractions: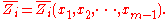
The partial molar property is thus an intensive property
Intensive and extensive properties
In the physical sciences, an intensive property , is a physical property of a system that does not depend on the system size or the amount of material in the system: it is scale invariant.By contrast, an extensive property In the physical sciences, an intensive property (also called a bulk...
- it does not depend on the size of the system.
Applications
Partial molar properties are useful because chemical mixtureMixture
In chemistry, a mixture is a material system made up by two or more different substances which are mixed together but are not combined chemically...
s are often maintained at constant temperature and pressure and under these conditions, the value of any extensive property
Intensive and extensive properties
In the physical sciences, an intensive property , is a physical property of a system that does not depend on the system size or the amount of material in the system: it is scale invariant.By contrast, an extensive property In the physical sciences, an intensive property (also called a bulk...
can be obtained from its partial molar property. They are especially useful when considering specific properties of pure substances (that is, properties of one mole of pure substance) and properties of mixing. By definition, properties of mixing are related to those of the pure substance by:
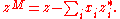
Here
 denotes the pure substance,
denotes the pure substance,  the mixing property, and
the mixing property, and  corresponds to the specific property under consideration. From the definition of partial molar properties,
corresponds to the specific property under consideration. From the definition of partial molar properties,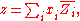
substitution yields:
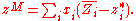
So from knowledge of the partial molar properties, properties of mixing can be calculated.
Relationship to thermodynamic potentials
Partial molar properties satisfy relations analogous to those of the extensive properties. For the internal energyInternal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
U, enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
H, Helmholtz free energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
A, and Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
G, the following hold:
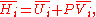
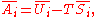
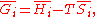
where
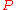 is the pressure,
is the pressure,  the volume
the volumeVolume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
,
 the temperature, and
the temperature, and 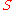 the entropy
the entropyEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
.
Differential form of the thermodynamic potentials
The thermodynamic potentials also satisfy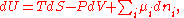
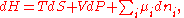
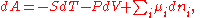
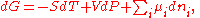
where
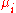 is the chemical potential
is the chemical potentialChemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
defined as (for constant nj with j≠i):

This last partial derivative
Partial derivative
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant...
is the same as
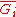 , the partial molar Gibbs free energy
, the partial molar Gibbs free energyGibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
. This means that the partial molar Gibbs free energy and the chemical potential, one of the most important properties in thermodynamics and chemistry, are the same quantity. Under isobaric
Isobaric process
An isobaric process is a thermodynamic process in which the pressure stays constant. The term derives from the Greek isos, , and barus,...
(constant P) and isothermal
Isothermal process
An isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir...
(constant T ) conditions, knowledge of the chemical potentials,
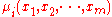 , yields every property of the mixture as they completely determine the Gibbs free energy.
, yields every property of the mixture as they completely determine the Gibbs free energy.Calculating partial molar properties
To calculate the partial molar property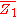 of a binary solution, one begins with the pure component denoted as
of a binary solution, one begins with the pure component denoted as 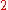 and, keeping the temperature and pressure constant during the entire process, add small quantities of component
and, keeping the temperature and pressure constant during the entire process, add small quantities of component 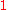 ; measuring
; measuring  after each addition. After sampling the compositions of interest one can fit a curve to the experimental data. This function will be
after each addition. After sampling the compositions of interest one can fit a curve to the experimental data. This function will be 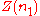 .
.Differentiating with respect to
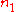 will give
will give 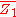 .
.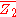 is then obtained from the relation:
is then obtained from the relation: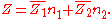
Further reading
- P. Atkins and J. de Paula, "Atkins' Physical Chemistry" (8th edition, Freeman 2006), chap.5
- T. Engel and P. Reid, "Physical Chemistry" (Pearson Benjamin-Cummings 2006), p.210
- K.J. Laidler and J.H. Meiser, "Physical Chemistry" (Benjamin-Cummings 1982), p.184-189
- P. Rock, "Chemical Thermodynamics" (MacMillan 1969), chap.9
External links
- Lecture notes from the University of Arizona detailing mixtures, partial molar quantities, and ideal solutions

