
Paleosalinity
Encyclopedia
Paleosalinity is the salinity of the global ocean or of an ocean basin at a point in geological history.
s, it is found that a decrease in the salinity of an aqueous fluid will act to increase the value of the carbon dioxide-carbonate system equilibrium constants, (pK*). This means that the relative proportion of carbonate
with respect to carbon dioxide is higher in more salinity fluids, e.g. seawater
, than in fresher waters. Of crucial importance for paleoclimatology
is the observation that an increase in salinity
will thus reduce the solubility
of carbon dioxide in the oceans. Since there is thought to have been a 120m depression in sea level at the last glacial maximum due to the extensive formation of ice sheet
s (which are solely freshwater), this represents a significant fractionation towards saltier seas during glacial periods. Correspondingly, this will cause a net outgassing of carbon dioxide into the atmosphere because of its reduced solubility, acting to increase atmospheric carbon dioxide by 6.5‰
. This is thought to partly offset the net decrease of 80-100‰ observed during glacial periods.
s effectively do no outcrop at the surface and are parallel to the surface. The ocean, in this case, can be described as "less ventilated", and this has been implicated in the slowing down of the MOC.
in pore fluids. Adkins et al. (2002) used pore fluid chlorinity in ODP
cores, with the paleo-depth estimated from nearby coral horizons. Chlorinity was measured rather than pure salinity because the major ions in seawater are not constant with depth in the sediment column; for example, sulphate reduction and cation-clay interactions can change overall salinity, whereas chlorinity is not heavily affected.
There are some notable differences in the hydrography at the LGM and present day. Today the North Atlantic Deep Water (NADW) is observed to be more saline than Antarctic Bottom Water (AABW), whereas at the last glacial maximum it was observed that the AABW was in fact more saline; a complete reversal. Today the NADW is more salty because of the Gulf Stream; this could thus indicate a reduction of flow through the Florida Straits due to lowered sea level.
Another observation is that the Southern Ocean was vastly more salty at the LGM than today, and noticeably more salty. This is particularly intriguiging given the assumed importance of the Southern Ocean
in oceanic dynamical regulation of ice ages. The extreme value of 37.1 psu is assumed to be a consequence of an increased degree of sea ice formation and export. This would account for the increased salinity, but would also account for the lack of oxygen isotopic fractionation; brine
rejection without oxygen isotopic fractionation is thought to be highly characteristic of sea ice formation.
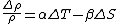
where is the thermal expansion coefficient and
is the thermal expansion coefficient and 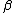 is the haline contraction coefficient. In particular, the ratio
is the haline contraction coefficient. In particular, the ratio  is crucial. Using the observed temperatures and salinities, in the modern ocean,
is crucial. Using the observed temperatures and salinities, in the modern ocean, 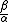 is about 10 whilst at the LGM
is about 10 whilst at the LGM 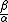 it is estimated to have been closer to 25. The modern thermohaline circulation
it is estimated to have been closer to 25. The modern thermohaline circulation
is thus more controlled by density contrasts due to thermal differences, whereas during the LGM the oceans were more than twice as sensitive to differences in salinity rather than temperature. In this way, the thermohaline circulation can be considered to have been less "thermo" and more "haline".
Importance
From Bjerrum plotBjerrum plot
A Bjerrum plot is a graph of concentrations of CO2, HCO3−, CO32−, and occasionally H+, and OH−, as functions of pH. They are typically used in ocean chemistry to track the response of an ocean to changes in both pH and of inputs in carbonate and CO2....
s, it is found that a decrease in the salinity of an aqueous fluid will act to increase the value of the carbon dioxide-carbonate system equilibrium constants, (pK*). This means that the relative proportion of carbonate
Carbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
with respect to carbon dioxide is higher in more salinity fluids, e.g. seawater
Seawater
Seawater is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% . This means that every kilogram of seawater has approximately of dissolved salts . The average density of seawater at the ocean surface is 1.025 g/ml...
, than in fresher waters. Of crucial importance for paleoclimatology
Paleoclimatology
Paleoclimatology is the study of changes in climate taken on the scale of the entire history of Earth. It uses a variety of proxy methods from the Earth and life sciences to obtain data previously preserved within rocks, sediments, ice sheets, tree rings, corals, shells and microfossils; it then...
is the observation that an increase in salinity
Salinity
Salinity is the saltiness or dissolved salt content of a body of water. It is a general term used to describe the levels of different salts such as sodium chloride, magnesium and calcium sulfates, and bicarbonates...
will thus reduce the solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
of carbon dioxide in the oceans. Since there is thought to have been a 120m depression in sea level at the last glacial maximum due to the extensive formation of ice sheet
Ice sheet
An ice sheet is a mass of glacier ice that covers surrounding terrain and is greater than 50,000 km² , thus also known as continental glacier...
s (which are solely freshwater), this represents a significant fractionation towards saltier seas during glacial periods. Correspondingly, this will cause a net outgassing of carbon dioxide into the atmosphere because of its reduced solubility, acting to increase atmospheric carbon dioxide by 6.5‰
Permille
A per mil or per mille is a tenth of a percent or one part per thousand. It is written with the sign ‰ , which looks like a percent sign with an extra zero at the end...
. This is thought to partly offset the net decrease of 80-100‰ observed during glacial periods.
Stratification
In addition, it is thought that extensive salinity stratification can lead to a reduction in the meridional overturning circulation (MOC) through the slowing of thermohaline circulation. Increased stratification means that there is effectively a barrier to subduction of parcels of water; isopycnalIsopycnal
An isopycnal is a surface of constant potential density of water. In the ocean, as the depth increases, so too does the density. Varying degrees of salinity and temperature act to modify the density of water, and the denser water always lies below the less dense water. Because of the action of...
s effectively do no outcrop at the surface and are parallel to the surface. The ocean, in this case, can be described as "less ventilated", and this has been implicated in the slowing down of the MOC.
Measuring Paleosalinity
There may exist proxies for salinity, but to date the main way that salinity has been measured has been by directly measuring chlorinityChlorinity
The chlorinity of water is defined as the mass of chlorine equivalent to the total mass of halogen contained in 1 kg seawater. It is determined by the Mohr-Knudsen titration, which however neglects fluoride....
in pore fluids. Adkins et al. (2002) used pore fluid chlorinity in ODP
Ocean Drilling Program
The Ocean Drilling Program was an international cooperative effort to explore and study the composition and structure of the Earth's ocean basins. ODP, which began in 1985, was the direct successor to the highly successful Deep Sea Drilling Project initiated in 1968 by the United States...
cores, with the paleo-depth estimated from nearby coral horizons. Chlorinity was measured rather than pure salinity because the major ions in seawater are not constant with depth in the sediment column; for example, sulphate reduction and cation-clay interactions can change overall salinity, whereas chlorinity is not heavily affected.
Paleosalinity during the Last Glacial Maximum
Adkins' study found that global salinity was greater, more or less as expected for a global sea level drop of 120m. By also analysing delta - O18 values, they also found that deep waters were within error of the freezing point of water, with oceanic waters exhibiting a greater degree of homogeneity in their temperatures. In contrast, variations in salinity were much greater than they are today. Modern day salinities are all within 0.5 psu of the global average salinity of 34.7 psu, whereas salinities during the last glacial maximum (LGM) ranged from 35.8 psu in the North Atlantic to 37.1 in the Southern Ocean.There are some notable differences in the hydrography at the LGM and present day. Today the North Atlantic Deep Water (NADW) is observed to be more saline than Antarctic Bottom Water (AABW), whereas at the last glacial maximum it was observed that the AABW was in fact more saline; a complete reversal. Today the NADW is more salty because of the Gulf Stream; this could thus indicate a reduction of flow through the Florida Straits due to lowered sea level.
Another observation is that the Southern Ocean was vastly more salty at the LGM than today, and noticeably more salty. This is particularly intriguiging given the assumed importance of the Southern Ocean
Southern Ocean
The Southern Ocean comprises the southernmost waters of the World Ocean, generally taken to be south of 60°S latitude and encircling Antarctica. It is usually regarded as the fourth-largest of the five principal oceanic divisions...
in oceanic dynamical regulation of ice ages. The extreme value of 37.1 psu is assumed to be a consequence of an increased degree of sea ice formation and export. This would account for the increased salinity, but would also account for the lack of oxygen isotopic fractionation; brine
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
rejection without oxygen isotopic fractionation is thought to be highly characteristic of sea ice formation.
The increased role of salinity
The presence of waters near the freezing point alters the balance of the relative effects of contrasts in salinity and temperature on sea water density. This is described in the equation,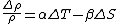
where
 is the thermal expansion coefficient and
is the thermal expansion coefficient and 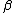 is the haline contraction coefficient. In particular, the ratio
is the haline contraction coefficient. In particular, the ratio  is crucial. Using the observed temperatures and salinities, in the modern ocean,
is crucial. Using the observed temperatures and salinities, in the modern ocean, 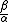 is about 10 whilst at the LGM
is about 10 whilst at the LGM 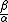 it is estimated to have been closer to 25. The modern thermohaline circulation
it is estimated to have been closer to 25. The modern thermohaline circulationThermohaline circulation
The term thermohaline circulation refers to a part of the large-scale ocean circulation that is driven by global density gradients created by surface heat and freshwater fluxes....
is thus more controlled by density contrasts due to thermal differences, whereas during the LGM the oceans were more than twice as sensitive to differences in salinity rather than temperature. In this way, the thermohaline circulation can be considered to have been less "thermo" and more "haline".

