Oxygen evolution
Encyclopedia
Oxygen evolution is the process of generating molecular oxygen
through chemical reaction
. Mechanisms of oxygen evolution include the oxidation of water
during oxygenic photosynthesis
, electrolysis
of water into oxygen and hydrogen
, and electrocatalytic oxygen evolution from oxide
s and oxoacid
s.
. The reaction is part of the light-dependent reactions of photosynthesis in cyanobacteria and the chloroplast
s of green algae
and plant
s. It utilizes the energy of light
to split a water molecule into its proton
s and electron
s for photosynthesis. Free oxygen is generated as a by-product of this reaction, and is released into the atmosphere
.
2H2O 4e- + 4H+ + O2
4e- + 4H+ + O2
The reaction requires the energy of four photon
s. The electrons from the oxidized water molecules replace electrons in the P680 component of photosystem II
that have been removed into an electron transport chain
via light-dependent excitation
and resonance energy transfer onto plastoquinone
. Photosytem II, therefore, has also been referred to as water-plastoquinone oxido-reductase. The protons are released into the thylakoid lumen, thus contributing to the generation of a proton gradient across the thylakoid membrane. This proton gradient is the driving force for ATP
synthesis via photophosphorylation
and coupling the absorption of light energy and oxidation of water to the creation of chemical energy during photosynthesis.
-containing cofactor
contained in photosystem II
known as the oxygen-evolving complex (OEC) or water-splitting complex. Manganese is an important cofactor
, and calcium
and chloride
are also required for the reaction to occur.
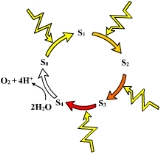
X-ray crystallography
studies have recently provided detailed models of the structure of the oxygen-evolving complex and its manganese cluster. Based on structural and spectroscopic
experiments, oxygen evolution involves a core three-plus-one cluster of three manganese ion
s and one calcium ion, with one additional manganese, which are oxidized via intermediate states called S-states. The O-O bond of molecular oxygen is formed between manganese-ligated oxygen atoms at the most oxidized, or S4, state.
evolved on earth around 3.5 billion years ago. Oxygen was not only a waste product of this reaction, but was also toxic to many metabolic processes such as nitrogen fixation
. As a consequence, it was released into the atmosphere as a means of detoxification
. This contributed to the conversion of earth's atmosphere from anaerobic to its current aerobic composition, triggering the oxygen catastrophe
and the evolution of aerobic metabolism
in utilizing the oxygen that was released by photosynthetic organisms as part of the oxygen cycle
.
discovered by accident the ability of plants to "restore" air that had been "injured" by the burning of a candle. He followed up on the experiment by showing that air "restored" by vegetation
was "not at all inconvenient to a mouse
." He was later awarded a medal for his discoveries that: "...no vegetable grows in vain... but cleanses and purifies our atmosphere." Priestley's experiments were followed up by Jan Ingenhousz
, a Dutch physician, who showed that "restoration" of air only worked in the presence of light and green plant parts.
Ingenhousz suggested in 1796 that CO2 (carbon dioxide
) is split during photosynthesis to release oxygen, while the carbon
combined with water to form carbohydrate
s. While this hypothesis was attractive and reasonable and thus widely accepted for a long time, it was later proven incorrect. Graduate student C.B. Van Niel at Stanford University
found that purple sulfur bacteria
reduce carbon to carbohydrates, but accumulate sulfur
instead of releasing oxygen. He boldly proposed that, in analogy to the sulfur bacteria's forming elemental sulfur from H2S (hydrogen sulfide
), plants would form oxygen from H2O (water). In 1937, this hypothesis was corroborated by the discovery that plants are capable of producing oxygen in the absence of CO2. This discovery was made by Robin Hill
, and subsequently the light-driven release of oxygen in the absence of CO2 was called the Hill reaction. Our current knowledge of the mechanism of oxygen evolution during photosynthesis was further established in experiments tracing isotopes of oxygen
from water to oxygen gas.
via electrolysis of water
. While oxygen production is not the main focus of industrial applications of water electrolysis, it becomes essential for life support system
s in situations that require the generation of oxygen for air revitalization. Human exploration of regions that lack breathable oxygen, such as the deep sea or outer space, requires means of reliably generating oxygen apart from earth's atmosphere. Submarine
s and spacecraft
utilize either an electrolytic
mechanism (water or solid oxide electrolysis) or chemical oxygen generator
s as part of their life support equipment.
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
through chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. Mechanisms of oxygen evolution include the oxidation of water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
during oxygenic photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
, electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of water into oxygen and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, and electrocatalytic oxygen evolution from oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s and oxoacid
Oxoacid
An oxoacid is an acid that contains oxygen. To be more specific, it is an acid that:#contains oxygen#contains at least one other element#has at least one hydrogen atom bound to oxygen#forms an ion by the loss of one or more protons....
s.
Oxygen evolution in nature
Photosynthetic oxygen evolution is the fundamental process by which breathable oxygen is generated in earth's biosphereBiosphere
The biosphere is the global sum of all ecosystems. It can also be called the zone of life on Earth, a closed and self-regulating system...
. The reaction is part of the light-dependent reactions of photosynthesis in cyanobacteria and the chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s of green algae
Green algae
The green algae are the large group of algae from which the embryophytes emerged. As such, they form a paraphyletic group, although the group including both green algae and embryophytes is monophyletic...
and plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s. It utilizes the energy of light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
to split a water molecule into its proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s and electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s for photosynthesis. Free oxygen is generated as a by-product of this reaction, and is released into the atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
.
Biochemical reaction
Photosynthetic oxygen evolution occurs via the light-dependent oxidation of water to molecular oxygen and can be written as the following simplified chemical reaction:2H2O
 4e- + 4H+ + O2
4e- + 4H+ + O2The reaction requires the energy of four photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s. The electrons from the oxidized water molecules replace electrons in the P680 component of photosystem II
Photosystem
Photosystems are functional and structural units of protein complexes involved in photosynthesis that together carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons...
that have been removed into an electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
via light-dependent excitation
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
and resonance energy transfer onto plastoquinone
Plastoquinone
Plastoquinone is a quinone molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. Plastoquinone is reduced , forming plastoquinol...
. Photosytem II, therefore, has also been referred to as water-plastoquinone oxido-reductase. The protons are released into the thylakoid lumen, thus contributing to the generation of a proton gradient across the thylakoid membrane. This proton gradient is the driving force for ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
synthesis via photophosphorylation
Photophosphorylation
The production of ATP using the energy of sunlight is called photophosphorylation. Only two sources of energy are available to living organisms: sunlight and reduction-oxidation reactions...
and coupling the absorption of light energy and oxidation of water to the creation of chemical energy during photosynthesis.
Oxygen-evolving complex
Water oxidation is catalyzed by a manganeseManganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
-containing cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
contained in photosystem II
Photosystem II
Photosystem II is the first protein complex in the Light-dependent reactions. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. The enzyme uses photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce...
known as the oxygen-evolving complex (OEC) or water-splitting complex. Manganese is an important cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
, and calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
and chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
are also required for the reaction to occur.
X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
studies have recently provided detailed models of the structure of the oxygen-evolving complex and its manganese cluster. Based on structural and spectroscopic
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
experiments, oxygen evolution involves a core three-plus-one cluster of three manganese ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s and one calcium ion, with one additional manganese, which are oxidized via intermediate states called S-states. The O-O bond of molecular oxygen is formed between manganese-ligated oxygen atoms at the most oxidized, or S4, state.
Evolution of oxygen evolution
Oxygen production during photosynthesisPhotosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
evolved on earth around 3.5 billion years ago. Oxygen was not only a waste product of this reaction, but was also toxic to many metabolic processes such as nitrogen fixation
Nitrogen fixation
Nitrogen fixation is the natural process, either biological or abiotic, by which nitrogen in the atmosphere is converted into ammonia . This process is essential for life because fixed nitrogen is required to biosynthesize the basic building blocks of life, e.g., nucleotides for DNA and RNA and...
. As a consequence, it was released into the atmosphere as a means of detoxification
Toxicity
Toxicity is the degree to which a substance can damage a living or non-living organisms. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell or an organ , such as the liver...
. This contributed to the conversion of earth's atmosphere from anaerobic to its current aerobic composition, triggering the oxygen catastrophe
Oxygen Catastrophe
The Great Oxygenation Event , also called the Oxygen Catastrophe or Oxygen Crisis or Great Oxidation, was the biologically induced appearance of free oxygen in Earth's atmosphere. This major environmental change happened around 2.4 billion years ago.Photosynthesis was producing oxygen both before...
and the evolution of aerobic metabolism
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
in utilizing the oxygen that was released by photosynthetic organisms as part of the oxygen cycle
Oxygen cycle
The Oxygen cycle is the biogeochemical cycle that describes the movement of oxygen within its three main reservoirs: the atmosphere , the total content of biological matter within the biosphere , and the lithosphere...
.
History of discovery
It was not until the end of the 18th century that Joseph PriestleyJoseph Priestley
Joseph Priestley, FRS was an 18th-century English theologian, Dissenting clergyman, natural philosopher, chemist, educator, and political theorist who published over 150 works...
discovered by accident the ability of plants to "restore" air that had been "injured" by the burning of a candle. He followed up on the experiment by showing that air "restored" by vegetation
Vegetation
Vegetation is a general term for the plant life of a region; it refers to the ground cover provided by plants. It is a general term, without specific reference to particular taxa, life forms, structure, spatial extent, or any other specific botanical or geographic characteristics. It is broader...
was "not at all inconvenient to a mouse
Mouse
A mouse is a small mammal belonging to the order of rodents. The best known mouse species is the common house mouse . It is also a popular pet. In some places, certain kinds of field mice are also common. This rodent is eaten by large birds such as hawks and eagles...
." He was later awarded a medal for his discoveries that: "...no vegetable grows in vain... but cleanses and purifies our atmosphere." Priestley's experiments were followed up by Jan Ingenhousz
Jan Ingenhousz
Jan Ingenhousz or Ingen-Housz FRS was a Dutch physiologist, biologist and chemist. He is best known for showing that light is essential to photosynthesis and thus having discovered photosynthesis. He also discovered that plants, like animals, have cellular respiration...
, a Dutch physician, who showed that "restoration" of air only worked in the presence of light and green plant parts.
Ingenhousz suggested in 1796 that CO2 (carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
) is split during photosynthesis to release oxygen, while the carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
combined with water to form carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s. While this hypothesis was attractive and reasonable and thus widely accepted for a long time, it was later proven incorrect. Graduate student C.B. Van Niel at Stanford University
Stanford University
The Leland Stanford Junior University, commonly referred to as Stanford University or Stanford, is a private research university on an campus located near Palo Alto, California. It is situated in the northwestern Santa Clara Valley on the San Francisco Peninsula, approximately northwest of San...
found that purple sulfur bacteria
Purple sulfur bacteria
The purple sulfur bacteria are a group of Proteobacteria capable of photosynthesis, collectively referred to as purple bacteria. They are anaerobic or microaerophilic, and are often found in hot springs or stagnant water. Unlike plants, algae, and cyanobacteria, they do not use water as their...
reduce carbon to carbohydrates, but accumulate sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
instead of releasing oxygen. He boldly proposed that, in analogy to the sulfur bacteria's forming elemental sulfur from H2S (hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
), plants would form oxygen from H2O (water). In 1937, this hypothesis was corroborated by the discovery that plants are capable of producing oxygen in the absence of CO2. This discovery was made by Robin Hill
Robin Hill (biochemist)
Robert Hill FRS , known as Robin Hill, was a British plant biochemist who, in 1939, demonstrated the 'Hill reaction' of photosynthesis, proving that oxygen is evolved during the light requiring steps of photosynthesis...
, and subsequently the light-driven release of oxygen in the absence of CO2 was called the Hill reaction. Our current knowledge of the mechanism of oxygen evolution during photosynthesis was further established in experiments tracing isotopes of oxygen
Isotopes of oxygen
There are three stable isotopes of oxygen that lead to oxygen having a standard atomic mass of 15.9994 u. 17 radioactive isotopes have also been characterized, with mass numbers from 12O to 28O, all short-lived, with the longest-lived being 15O with a half-life of 122.24 seconds...
from water to oxygen gas.
Technological oxygen evolution
Oxygen evolution occurs as a byproduct of hydrogen productionHydrogen production
Hydrogen production is the family of industrial methods for generating hydrogen. Currently the dominant technology for direct production is steam reforming from hydrocarbons. Many other methods are known including electrolysis and thermolysis...
via electrolysis of water
Electrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
. While oxygen production is not the main focus of industrial applications of water electrolysis, it becomes essential for life support system
Life support system
In human spaceflight, a life support system is a group of devices that allow a human being to survive in space.US government space agency NASA,and private spaceflight companies...
s in situations that require the generation of oxygen for air revitalization. Human exploration of regions that lack breathable oxygen, such as the deep sea or outer space, requires means of reliably generating oxygen apart from earth's atmosphere. Submarine
Submarine
A submarine is a watercraft capable of independent operation below the surface of the water. It differs from a submersible, which has more limited underwater capability...
s and spacecraft
Spacecraft
A spacecraft or spaceship is a craft or machine designed for spaceflight. Spacecraft are used for a variety of purposes, including communications, earth observation, meteorology, navigation, planetary exploration and transportation of humans and cargo....
utilize either an electrolytic
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
mechanism (water or solid oxide electrolysis) or chemical oxygen generator
Chemical oxygen generator
A chemical oxygen generator is a device releasing oxygen created by a chemical reaction. The oxygen source is usually an inorganic superoxide, chlorate, or perchlorate. A promising group of oxygen sources are ozonides. The generators are usually ignited mechanically, by a firing pin, and the...
s as part of their life support equipment.
External links
- Plant Physiology Online, 4th edition: Topic 7.7 - Oxygen Evolution
- Oxygen evolution - Lecture notes by Antony Crofts, UIUC
- Evolution of the atmosphere – Lecture notes, Regents of the University of Michigan
- How to make oxygen and hydrogen from water using electrolysis

