Oxo Diels Alder reaction
Encyclopedia
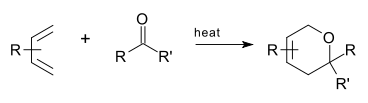
An Oxo Diels–Alder reaction is an organic reaction
and a variation of the Diels-Alder reaction
in which a suitable diene
reacts with an aldehyde
to form a dihydropyran ring. This reaction is of some importance to synthetic organic chemistry.
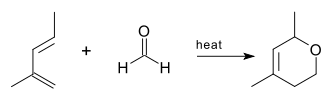
The oxo DA reaction was first reported in 1949 using a methylpentadiene and formaldehyde
as reactants.
Asymmetric oxo-DA reactions (including catalytic reactions) are well known. Many strategies rely on coordinating a chiral Lewis acid
to the carbonyl group.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
and a variation of the Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
in which a suitable diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
reacts with an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
to form a dihydropyran ring. This reaction is of some importance to synthetic organic chemistry.
The oxo DA reaction was first reported in 1949 using a methylpentadiene and formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
as reactants.
Asymmetric oxo-DA reactions (including catalytic reactions) are well known. Many strategies rely on coordinating a chiral Lewis acid
Chiral Lewis acid
Chiral Lewis acids are a novel class of Lewis acid catalyst used in enantioselective asymmetric synthesis reactions which produce optically active products from optically inactive or impure starting materials. This type of preferential formation of one enantiomer or diastereomer over the other is...
to the carbonyl group.