Oxaloacetic acid
Encyclopedia
Oxaloacetic acid is an organic compound
with the chemical formula C4H4O5 or HOOC-(C=O)-(CH2)-COOH. It also has other names (see the table).
Its fully deprotonated
derivative is the oxaloacetate anion, C4H2O52− or [(C=O)2(CH2)(C=O)]2−; this name is also used for esters that contain the divalent
[-O(C=O)2(CH2)(C=O)O-] moiety
. Loss of a single proton gives the acid's conjugate base, the anion hydrogenoxaloacetate anion H(C=O)2(CH2)(C=O)−.
dicarboxylic acid
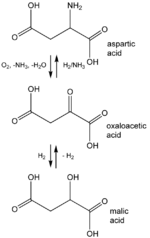
is a protonated variant of oxaloacetate, which is an intermediate of the citric acid cycle
and gluconeogenesis
. Oxaloacetate forms upon oxidation of L-malate
, catalysed by malate dehydrogenase
, and reacts with Acetyl-CoA
to form citrate
, catalysed by citrate synthase
. It also forms in the mesophyll
of plants by the condensation of CO2
with phosphoenolpyruvate
, catalysed by PEP Carboxykinase
. It can arise from pyruvate via an anaplerotic reaction
. Oxaloacetate is also a potent inhibitor of Complex II.
forms of oxaloacetic acid are particularly stable, so much so that the two isomers have different melting points (152 °C cis, 184 °C trans). The enol proton has a pKa value
of 13.02.
Oxaloacetate is unstable in solution, decomposing to pyruvate by decarboxylation
over a period of hours (room temperature) or days (0 °C). Refrigerated storage of the solid is therefore recommended.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the chemical formula C4H4O5 or HOOC-(C=O)-(CH2)-COOH. It also has other names (see the table).
Its fully deprotonated
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
derivative is the oxaloacetate anion, C4H2O52− or [(C=O)2(CH2)(C=O)]2−; this name is also used for esters that contain the divalent
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
[-O(C=O)2(CH2)(C=O)O-] moiety
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
. Loss of a single proton gives the acid's conjugate base, the anion hydrogenoxaloacetate anion H(C=O)2(CH2)(C=O)−.
Function
This four-carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
dicarboxylic acid
Dicarboxylic acid
Dicarboxylic acids are organic compounds that contain two carboxylic acid functional groups. In molecular formulae for dicarboxylic acids, these groups are often written as HOOC-R-COOH, where R may be an alkyl, alkenyl, alkynyl, or aryl group...
is a protonated variant of oxaloacetate, which is an intermediate of the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
and gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
. Oxaloacetate forms upon oxidation of L-malate
Malic acid
Malic acid is an organic compound with the formula HO2CCH2CHOHCO2H. It is a dicarboxylic acid which is made by all living organisms, contributes to the pleasantly sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms , though only the L-isomer exists...
, catalysed by malate dehydrogenase
Malate dehydrogenase
Malate dehydrogenase is an enzyme in the citric acid cycle that catalyzes the conversion of malate into oxaloacetate and vice versa...
, and reacts with Acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
to form citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
, catalysed by citrate synthase
Citrate synthase
The enzyme citrate synthase exists in nearly all living cells and stands as a pace-making enzyme in the first step of the Citric Acid Cycle . Citrate synthase is localized within eukaryotic cells in the mitochondrial matrix, but is encoded by nuclear DNA rather than mitochondrial...
. It also forms in the mesophyll
Mesophyll
Mesophyll can refer to:* Mesophyll tissue, in plant anatomy, photosynthetic parenchyma cells that lie between the upper and lower epidermis layers of a leaf...
of plants by the condensation of CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
with phosphoenolpyruvate
Phosphoenolpyruvate
Phosphoenolpyruvic acid , or phosphoenolpyruvate as the anion, is an important chemical compound in biochemistry. It has the high-energy phosphate bond found in living organisms, and is involved in glycolysis and gluconeogenesis...
, catalysed by PEP Carboxykinase
Phosphoenolpyruvate carboxykinase
Phosphoenolpyruvate carboxykinase is an enzyme in the lyase family used in the metabolic pathway of gluconeogenesis. It converts oxaloacetate into phosphoenolpyruvate and carbon dioxide.It is found in two forms, cytosolic and mitochondrial....
. It can arise from pyruvate via an anaplerotic reaction
Anaplerotic reactions
Anaplerotic reactions are those that form intermediates of a metabolic pathway. Examples of such are found in the Tricarboxylic acid Cycle...
. Oxaloacetate is also a potent inhibitor of Complex II.

Chemical properties
The enolEnol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
forms of oxaloacetic acid are particularly stable, so much so that the two isomers have different melting points (152 °C cis, 184 °C trans). The enol proton has a pKa value
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of 13.02.
Oxaloacetate is unstable in solution, decomposing to pyruvate by decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
over a period of hours (room temperature) or days (0 °C). Refrigerated storage of the solid is therefore recommended.

