
Osmotic pressure
Encyclopedia
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
which needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane
Semipermeable membrane
A semipermeable membrane, also termed a selectively permeable membrane, a partially permeable membrane or a differentially permeable membrane, is a membrane that will allow certain molecules or ions to pass through it by diffusion and occasionally specialized "facilitated diffusion".The rate of...
.
The phenomenon of osmotic pressure arises from the tendency of a pure solvent to move through a semi-permeable membrane and into a solution containing a solute to which the membrane is impermeable. This process is of vital importance in biology as the cell's membrane
Biological membrane
A biological membrane or biomembrane is an enclosing or separatingmembrane that acts as a selective barrier, within or around a cell. It consists of a lipid bilayer with embedded proteins that may constitute close to 50% of membrane content...
is selective towards many of the solutes found in living organisms.
In order to visualize this effect, imagine a U-shaped clear tube with equal amounts of water on each side, separated by a membrane at its base that is impermeable to sugar molecules (made from dialysis tubing
Dialysis tubing
Dialysis Tubing or visking tubing is a type of semi- or partially permeable membrane tubing made from regenerated cellulose or cellophane. It can be used for diffusion with solutes or osmosis if used with water only. Osmosis is when water passes through a semi-permeable layer to reach equilibrium...
). Sugar has been added to the water on one side. The height of the water on each side will change proportional to the pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
of the solutions.
Osmotic pressure causes the height of the water in the compartment containing the sugar to rise, due to movement of the pure water from its compartment into the compartment containing the sugar water. This process will stop once the pressures of the water and sugar water toward both sides of the membrane are equated. (See Osmosis
Osmosis
Osmosis is the movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, aiming to equalize the solute concentrations on the two sides...
).
Jacobus Henricus van 't Hoff
Jacobus Henricus van 't Hoff
Jacobus Henricus van 't Hoff, Jr. was a Dutch physical and organic chemist and the first winner of the Nobel Prize in chemistry. He is best known for his discoveries in chemical kinetics, chemical equilibrium, osmotic pressure, and stereochemistry...
first proposed a formula for calculating the osmotic pressure, but this was later improved upon by Harmon Northrop Morse
Harmon Northrop Morse
Harmon Northrop Morse was an American chemist. Today he is known as the first to have synthesized paracetamol, but this substance only became widely used as a drug decades after Morse's death. In the first half of the 20th century he was best known for his study of osmotic pressure, for which he...
.
Osmotic potential is the opposite of water potential
Water potential
Water potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure, or matrix effects such as surface tension...
, which is the degree to which a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
tends to stay in a liquid.
Thermodynamic explanation
Consider the system at the point it has reached equilibrium. The condition for this is that the chemical potentialChemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
of the solvent (since only it is free to flow towards equilibrium) on both sides of the membrane is equal. The compartment containing the pure solvent has a chemical potential of
 . On the other side, the compartment containing the solute has an additional contribution from the solute (factored as the mole fraction of the solute,
. On the other side, the compartment containing the solute has an additional contribution from the solute (factored as the mole fraction of the solute,  < 1) but there also appears an addition in pressure. The balance is therefore:
< 1) but there also appears an addition in pressure. The balance is therefore: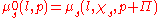
where p denotes the external pressure, l the solvent,
 the mole fraction of the solvent and
the mole fraction of the solvent and  the osmotic pressure exerted by the solutes. The addition of solute decreases the chemical potential (an entropic effect
the osmotic pressure exerted by the solutes. The addition of solute decreases the chemical potential (an entropic effectEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
), while the pressure increases the chemical potential, and thus a balance is reached.
Note that the presence of the solute decreases the potential due to
 being smaller than 1.
being smaller than 1.Derivation of osmotic pressure
In order to find , the osmotic pressure, we can write the chemical potentials explicitly:
, the osmotic pressure, we can write the chemical potentials explicitly: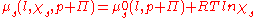
The negative expression on the left is a result of the increase in available states, causing an increase in entropy and a reduction of the chemical potential. The addition to the pressure is expressed through the expression for the energy of expansion:
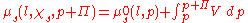
Inserting the expression presented above into the chemical potential equation for the entire system and rearranging will arrive at:
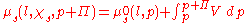
Morse equation
The osmotic pressure Π of a dilute solution can be approximated using the Morse equation (named after Harmon Northrop MorseHarmon Northrop Morse
Harmon Northrop Morse was an American chemist. Today he is known as the first to have synthesized paracetamol, but this substance only became widely used as a drug decades after Morse's death. In the first half of the 20th century he was best known for his study of osmotic pressure, for which he...
):
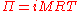 ,
,where
- i is the dimensionlessDimensionless quantityIn dimensional analysis, a dimensionless quantity or quantity of dimension one is a quantity without an associated physical dimension. It is thus a "pure" number, and as such always has a dimension of 1. Dimensionless quantities are widely used in mathematics, physics, engineering, economics, and...
van 't Hoff factorVan 't Hoff factorThe van 't Hoff factor i is a measure of the effect of a solute upon colligative properties, such as vapor pressure, osmotic pressure and freezing point depression. The van 't Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved, and the... - M is the molarity
- R=0.0821 L atm K-1 mol-1 is the gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
- T is the thermodynamic (absolute) temperatureThermodynamic temperatureThermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
This equation gives the pressure on one side of the membrane; the total pressure on the membrane is given by the difference between the pressures on the two sides. Note the similarity of the above formula to the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
and also that osmotic pressure is not dependent on particle charge. This equation was derived by van 't Hoff
Jacobus Henricus van 't Hoff
Jacobus Henricus van 't Hoff, Jr. was a Dutch physical and organic chemist and the first winner of the Nobel Prize in chemistry. He is best known for his discoveries in chemical kinetics, chemical equilibrium, osmotic pressure, and stereochemistry...
.
Osmotic pressure is an important factor affecting cells. Osmoregulation
Osmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's fluids to maintain the homeostasis of the organism's water content; that is it keeps the organism's fluids from becoming too diluted or too concentrated. Osmotic pressure is a measure of the tendency of water to move...
is the homeostasis
Homeostasis
Homeostasis is the property of a system that regulates its internal environment and tends to maintain a stable, constant condition of properties like temperature or pH...
mechanism of an organism to reach balance in osmotic pressure.
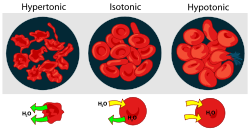
- Hypertonicity is the presence of a solution that causes cells to shrink.
- Hypotonicity is the presence of a solution that causes cells to swell.
- Isotonic is the presence of a solution that produces no change in cell volume.
When a biological
Biological tissue
Tissue is a cellular organizational level intermediate between cells and a complete organism. A tissue is an ensemble of cells, not necessarily identical, but from the same origin, that together carry out a specific function. These are called tissues because of their identical functioning...
cell
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
is in a hypotonic environment, the cell interior accumulates water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, water flows across the cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
into the cell, causing it to expand. In plant cell
Plant cell
Plant cells are eukaryotic cells that differ in several key respects from the cells of other eukaryotic organisms. Their distinctive features include:...
s, the cell wall
Cell wall
The cell wall is the tough, usually flexible but sometimes fairly rigid layer that surrounds some types of cells. It is located outside the cell membrane and provides these cells with structural support and protection, and also acts as a filtering mechanism. A major function of the cell wall is to...
restricts the expansion, resulting in pressure on the cell wall from within called turgor pressure
Turgor pressure
Turgor Pressure or turgidity is the main pressure of the cell contents against the cell wall in plant cells and bacteria cells, determined by the water content of the vacuole, resulting from osmotic pressure, i.e...
.
Applications
Osmotic pressure is the basis of filtering ("reverse osmosisReverse osmosis
Reverse osmosis is a membrane technical filtration method that removes many types of large molecules and ions from solutions by applying pressure to the solution when it is on one side of a selective membrane. The result is that the solute is retained on the pressurized side of the membrane and...
"), a process commonly used to purify water. The water to be purified is placed in a chamber and put under an amount of pressure greater than the osmotic pressure exerted by the water and the solutes dissolved in it. Part of the chamber opens to a differentially permeable membrane that lets water molecules through, but not the solute particles. The osmotic pressure of ocean water is about 27 atm
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
. Reverse osmosis desalinates
Desalination
Desalination, desalinization, or desalinisation refers to any of several processes that remove some amount of salt and other minerals from saline water...
fresh water from ocean salt water
Seawater
Seawater is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% . This means that every kilogram of seawater has approximately of dissolved salts . The average density of seawater at the ocean surface is 1.025 g/ml...
.
Osmotic pressure is necessary for many plant functions. It is the resulting turgor pressure
Turgor pressure
Turgor Pressure or turgidity is the main pressure of the cell contents against the cell wall in plant cells and bacteria cells, determined by the water content of the vacuole, resulting from osmotic pressure, i.e...
on the cell wall that allows herbaceous plant
Herbaceous plant
A herbaceous plant is a plant that has leaves and stems that die down at the end of the growing season to the soil level. They have no persistent woody stem above ground...
s to stand upright, and how plants regulate the aperture of their stomata. In animal cells which lack a cell wall however, excessive osmotic pressure can result in cytolysis
Cytolysis
Cytolysis, or osmotic lysis, occurs when a cell bursts due to an osmotic imbalance that has caused excess water to move into the cell. It occurs in a hypotonic environment, where water diffuses into the cell and causes its volume to increase. If the volume of water exceeds the cell membrane's...
.
- Cell wallCell wallThe cell wall is the tough, usually flexible but sometimes fairly rigid layer that surrounds some types of cells. It is located outside the cell membrane and provides these cells with structural support and protection, and also acts as a filtering mechanism. A major function of the cell wall is to...
- CytolysisCytolysisCytolysis, or osmotic lysis, occurs when a cell bursts due to an osmotic imbalance that has caused excess water to move into the cell. It occurs in a hypotonic environment, where water diffuses into the cell and causes its volume to increase. If the volume of water exceeds the cell membrane's...
- Gibbs-Donnan effectGibbs-Donnan effectThe Gibbs–Donnan effect is a name for the behavior of charged particles near a semi-permeable membrane to sometimes fail to distribute evenly across the two sides of the membrane...
- OsmosisOsmosisOsmosis is the movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, aiming to equalize the solute concentrations on the two sides...
- Pfeffer cellWilhelm PfefferWilhelm Friedrich Philipp Pfeffer was a German botanist and plant physiologist who was born in Grebenstein.- Academic career :...
- PlasmolysisPlasmolysisPlasmolysis is the process in plant cells where the cytoplasm pulls away from the cell wall due to the loss of water through osmosis. The reverse process, cytolysis, can occur if the cell is in a hypotonic solution resulting in a higher external osmotic pressure and a net flow of water into the cell...
- Turgor pressureTurgor pressureTurgor Pressure or turgidity is the main pressure of the cell contents against the cell wall in plant cells and bacteria cells, determined by the water content of the vacuole, resulting from osmotic pressure, i.e...
For the calculation of molecular weight by using colligative properties, osmotic pressure is the most preferred property.

