Oseltamivir total synthesis
Encyclopedia
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of the antiinfluenza drug oseltamivir
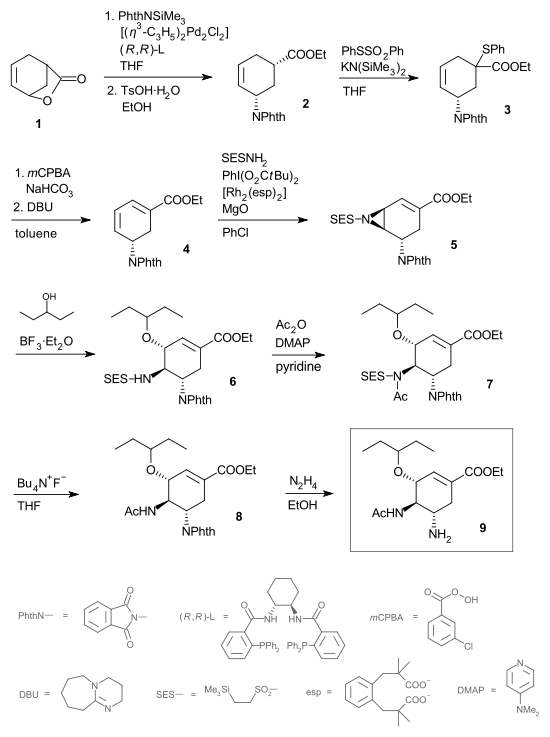
Oseltamivir
Oseltamivir INN , an antiviral drug, slows the spread of influenza virus between cells in the body by stopping the virus from chemically cutting ties with its host cell; median time to symptom alleviation is reduced by 0.5–1 day. The drug is sold under the trade name Tamiflu, and is taken orally...
marketed by Hoffmann-La Roche
Hoffmann-La Roche
F. Hoffmann-La Roche Ltd. is a Swiss global health-care company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX Swiss Exchange....
under the trade name
Trade name
A trade name, also known as a trading name or a business name, is the name which a business trades under for commercial purposes, although its registered, legal name, used for contracts and other formal situations, may be another....
Tamiflu. Its commercial production starts from the biomolecule
Biomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
shikimic acid
Shikimic acid
Shikimic acid, more commonly known as its anionic form shikimate, is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi , from which it was first isolated....
harvested from Chinese star anise
Star anise
Illicium verum, commonly called Star anise, star aniseed, or Chinese star anise, is a spice that closely resembles anise in flavor, obtained from the star-shaped pericarp of Illicium verum, a small native evergreen tree of northeast Vietnam and southwest China...
with a limited worldwide supply. Due to its limited supply, searches for alternative synthetic routes preferably skipping shikimic acid are underway and to date several such routes have been published. Control of stereochemistry is important: the molecule has three stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s and the sought-after isomer is only 1 of 8 stereoisomers.
Commercial production
The current production method is based on the first scalable synthesis developed by Gilead SciencesGilead Sciences
Gilead Sciences is a biopharmaceutical company that discovers, develops and commercializes therapeutics. For many years since the company was founded, the company concentrated primarily on antiviral drugs to treat patients infected with HIV, hepatitis B or influenza. In 2006, Gilead acquired two...
starting from naturally occurring quinic acid
Quinic acid
Quinic acid is a cyclitol, a cyclic polyol. It is a crystalline acid obtained from cinchona bark, coffee beans, and other plant products and made synthetically by hydrolysis of chlorogenic acid. Quinic acid is also implicated in the perceived acidity of coffee...
or shikimic acid
Shikimic acid
Shikimic acid, more commonly known as its anionic form shikimate, is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi , from which it was first isolated....
. Due to lower yields and the extra steps required (because of the additional dehydration), the quinic acid route was dropped in favour of the one based on shikimic acid, which received further improvements by Hoffmann-La Roche
Hoffmann-La Roche
F. Hoffmann-La Roche Ltd. is a Swiss global health-care company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX Swiss Exchange....
.
The current industrial synthesis is summarised below:
Karpf / Trussardi synthesis
The current production method includes two reaction steps with potentially hazardous azideAzide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
s. A reported azide-free Roche synthesis of tamiflu is summarised graphically below :

Shikimic acid
Shikimic acid, more commonly known as its anionic form shikimate, is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi , from which it was first isolated....
. The 3,4-pentylidene acetal mesylate
Mesylate
In chemistry, a mesylate is any salt or ester of methanesulfonic acid . In salts, the mesylate is present as the CH3SO3− anion. When modifying the International Nonproprietary Name of a pharmaceutical substance containing the group or anion, the correct spelling is mesilate .Mesylate esters are a...
is prepared in three steps: esterification with ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
; ketalization with p-toluenesulfonic acid
P-Toluenesulfonic acid
p-Toluenesulfonic acid or tosylic acid is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as the Tosyl group and is often abbreviated as Ts or Tos...
and 3-pentanone
3-Pentanone
3-Pentanone is a simple, symmetrical dialkyl ketone. It is a colorless liquid ketone with an odor like that of acetone. It is soluble in about 25 parts water, but miscible with organic solvents. It is mainly used as a solvent in paint and a precursor to vitamin E...
; and mesylation with triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
and methanesulfonyl chloride
Methanesulfonyl chloride
Methanesulfonyl chloride is a compound containing a sulfonyl chloride used to make methanesulfonates and to generate sulfene.-Preparation, manufacture and handling:Methanesulfonyl chloride is highly toxic, moisture sensitive, corrosive, and a lachrymator...
. Reductive opening of the ketal under modified Hunter conditions in dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
yields an inseparable mixture of isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
ic mesylates. The corresponding epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
is formed under basic conditions with potassium bicarbonate
Potassium bicarbonate
Potassium bicarbonate , is a colorless, odorless, slightly basic, salty substance...
. Using the inexpensive Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
magnesium bromide diethyl etherate (commonly prepared fresh by the addition of magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
turnings to 1,2-dibromoethane
1,2-Dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide , is the chemical compound with the formula BrCH2CH2Br. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly a synthetic...
in benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
:diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
), the epoxide is opened with allyl amine to yield the corresponding 1,2-amino alcohol. The water-immiscible solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s methyl tert-butyl ether
Methyl tert-butyl ether
Methyl tert-butyl ether, also known as methyl tertiary butyl ether and MTBE, is an organic compound with molecular formula 3COCH3. MTBE is a volatile, flammable, and colorless liquid that is immiscible with water. It has a minty odor vaguely reminiscent of diethyl ether, leading to unpleasant taste...
and acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
are used to simplify the workup procedure, which involved stirring with 1 M aqueous ammonium sulfate
Ammonium sulfate
Ammonium sulfate , 2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen as ammonium cations, and 24% sulfur as sulfate anions...
. Reduction on palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
, promoted by ethanolamine
Ethanolamine
Ethanolamine, also called 2-aminoethanol or monoethanolamine , is an organic chemical compound that is both a primary amine and a primary alcohol . Like other amines, monoethanolamine acts as a weak base...
, followed by acidic workup yielded the deprotected 1,2-aminoalcohol. The aminoalcohol was converted directly to the corresponding allyl-diamine in an interesting cascade sequence that commences with the unselective imination of benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
with azeotropic water removal in methyl tert-butyl ether. Mesylation, followed by removal of the solid byproduct triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
hydrochloride
Hydrochloride
In chemistry, hydrochlorides are salts resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base . This is also known as muriate, derived from hydrochloric acid's other name: muriatic acid....
, results in an intermediate that was poised to undergo aziridination upon transimination with another equivalent of allylamine. With the librated methanesulfonic acid
Methanesulfonic acid
Methanesulfonic acid is a colorless liquid with the chemical formula CH3SO3H. It is the simplest of the alkylsulfonic acids. Salts and esters of methanesulfonic acid are known as mesylates. Methanesulfonic acid is used as an acid catalyst in organic reactions because it is non-volatile, strong acid...
, the
aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
opens cleanly to yield a diamine that immediately undergoes a second transimination. Acidic hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
then removed the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
. Selective acylation
Acylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
with acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
(under buffered
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
conditions, the 5-amino group is protonated
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
owing to a considerable difference in pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
, 4.2 vs 7.9, preventing acetylation
Acetylation
Acetylation describes a reaction that introduces an acetyl functional group into a chemical compound...
) yields the desired N-acetylated product in crystalline form upon extractive workup. Finally, deallylation as above, yielded the freebase
Freebase (chemistry)
Freebase or free base refers to the pure basic form of an amine, as opposed to its salt form. The amine is usually an alkaloid natural product. Free base is commonly used in organic chemistry and pharmaceuticals to describe the unprotonated amine form of a compound.Most alkaloids are unstable in...
of oseltamivir, which was converted to the desired oseltamivir phosphate by treatment with phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
. The final product is obtained in high purity (99.7%) and an overall yield of 17-22% from (−)-shikimic acid. It is noted that the synthesis avoids the use of potentially explosive azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
reagents and intermediates; however, the synthesis actually used by Roche uses azides. Roche has other routes to
oseltamivir that do not involve the use of (−)-shikimic acid as a chiral pool starting material, such as a Diels-Alder route involving furan and ethyl acrylate
Ethyl acrylate
Ethyl acrylate is an organic compound primarily used in the preparation of various polymers. It is a clear liquid with an acrid penetrating odor. The human nose is capable of detecting this odor at a thousand times lower concentration than is considered harmful if continuously exposed for some...
or an isophthalic acid
Isophthalic acid
Isophthalic acid is an organic compound with the formula C6H42. This colourless solid is an isomer of phthalic acid and terephthalic acid. These aromatic dicarboxylic acids are used as precursors to commercially important polymers, e.g. the fire-resistant material Nomex...
route, which involves catalytic hydrogenation and enzymatic desymmetrization.
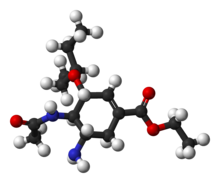
Corey synthesis
In 2006 the group of E.J. Corey published a novel route bypassing shikimic acid starting from butadiene and acrylic acidAcrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
. The inventors chose not to patent
Patent
A patent is a form of intellectual property. It consists of a set of exclusive rights granted by a sovereign state to an inventor or their assignee for a limited period of time in exchange for the public disclosure of an invention....
this procedure which is described below.

Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
with the esterfication product of acrylic acid
Acrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
and 2,2,2-Trifluoroethanol
2,2,2-Trifluoroethanol
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic...
2 catalysed by the CBS catalyst
CBS catalyst
The CBS catalyst or Corey-Bakshi-Shibata catalyst is an asymmetric catalyst derived from proline. It finds many uses in organic reactions such as the CBS reduction, Diels-Alder reactions and [3+2] cycloadditions. Proline, a naturally occurring chiral compound, is readily and cheaply available...
. The ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
3 is converted into an amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
in 4 by reaction with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
and the next step to lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
5 is an iodolactamization with iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
initiated by trimethylsilyltriflate. The amide group is fitted with a BOC protective group by reaction with Boc anhydride in 6 and the iodine substituent is removed in an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
with DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
to the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
7. Bromine is introduced in 8 by an allylic bromination with NBS
N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
and the amide group is cleaved with ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and caesium carbonate
Caesium carbonate
Caesium carbonate is a white crystalline solid of formula Cs2CO3. It is more soluble in organic solvents than many other carbonates such as potassium carbonate, and therefore finds use as a base in organic chemistry....
accompanied by elimination of bromide to the diene ethyl ester 9. The newly formed double bond is functionalized with N-bromoacetamide
Acetamide
Acetamide is an organic compound with the formula CH3CONH2. It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent...
10 catalyzed with
Tin(IV) bromide
Tin(IV) bromide
Tin bromide is the chemical compound SnBr4. It is a colourless low melting solid.SnBr4 can be prepared by reaction of the elements at normal temperatures:In aqueous solution Sn64+ is the principal ionic species amongst a range of 6 coordinate ions with from 0-6 bromide ligands Tin(IV) bromide is...
with complete control of stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
. In the next step the bromine atom in 11 is displaced by the nitrogen atom in the amide group with the strong base KHMDS
Sodium bis(trimethylsilyl)amide
Sodium bisamide is the chemical compound with the formula 2NNa. This species, usually called NaHMDS , is a strong base used for deprotonation reactions or base catalyzed reaction...
to the aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
12 which in turn is opened by reaction with 3-pentanol 13 to the ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
14. In the final step the BOC group is removed with phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
and the oseltamivir phosphate 15 is formed.
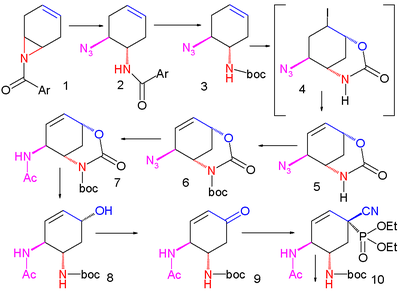
Shibasaki synthesis
Also in 2006 the group of Masakatsu Shibasaki of the University of TokyoUniversity of Tokyo
, abbreviated as , is a major research university located in Tokyo, Japan. The University has 10 faculties with a total of around 30,000 students, 2,100 of whom are foreign. Its five campuses are in Hongō, Komaba, Kashiwa, Shirokane and Nakano. It is considered to be the most prestigious university...
published a synthesis again bypassing shikimic acid .
 |
 |
|
| Shibasaki Tamiflu synthesis Part I | Part II | |
An improved method published in 2007 starts with the enantioselective desymmetrization
Desymmetrization
Desymmetrization in stereochemistry is the modification of a molecule that results in the loss of one or more symmetry elements. An important application involves the introduction of chirality. Formally, such conversion require the loss of an improper axis of rotation...
of aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
1 with trimethylsilyl azide
Trimethylsilyl azide
Trimethylsilyl azide is a chemical compound used as a reagent in organic chemistry.-Preparation:Trimethylsilyl azide is commercially available. It may be prepared by the reaction of trimethylsilyl chloride and sodium azide:-Applications:...
(TMSN3) and a chiral catalyst to the azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
2. The amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
group is protected as a BOC group with Boc anhydride and DMAP in 3 and iodolactamization with iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
and potassium carbonate
Potassium carbonate
Potassium carbonate is a white salt, soluble in water , which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It is deliquescent, often appearing a damp or wet solid...
first gives the unstable intermediate 4 and then stable cyclic carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
5 after elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of hydrogen iodide
Hydrogen iodide
Hydrogen iodide is a diatomic molecule. Aqueous solutions of HI are known as iohydroic acid or hydroiodic acid, a strong acid. Gas and aqueous solution are interconvertible...
with DBU
DBU (chemistry)
1,8-Diazabicyclo[5.4.0]undec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst and complexing ligand and a strong non-nucleophilic base.It is used as a curing agent for epoxy; it is used as a protecting...
.
The amide group is reprotected as BOC 6 and the azide group converted to the amide 7 by reductive acylation with thioacetic acid
Thioacetic acid
Thioacetic acid is an organosulfur compound with the molecular formula CH3COSH. It is a colourless liquid with a strong thiol-like odor. It is used in organic synthesis for the introduction of thiol groups in molecules-Synthesis and properties:...
and 2,6-lutidine
2,6-Lutidine
2,6-Lutidine is a natural heterocyclic aromatic organic compound. It has been isolated from the basic fraction of coal tar and from bone oil. It is a dimethyl substituted derivative of pyridine. It has been detected in waste water from oil shale processing sites and former creosoting facilities...
. Caesium carbonate
Caesium carbonate
Caesium carbonate is a white crystalline solid of formula Cs2CO3. It is more soluble in organic solvents than many other carbonates such as potassium carbonate, and therefore finds use as a base in organic chemistry....
accomplishes the hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the carbamate group to the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
8 which is subsequently oxidized to ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
9 with Dess-Martin periodinane
Dess-Martin periodinane
Dess–Martin periodinane is a chemical reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions , shorter reaction times, higher yields, simplified workups,...
. Cyanophosphorylation with diethyl phosphorocyanidate (DEPC) modifies the ketone group to the cyanophosphate 10 paving the way for an intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
allylic rearrangement
Allylic rearrangement
An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution....
to unstable β-allyl phosphate
Organophosphate
An organophosphate is the general name for esters of phosphoric acid. Phosphates are probably the most pervasive organophosphorus compounds. Many of the most important biochemicals are organophosphates, including DNA and RNA as well as many cofactors that are essential for life...
11 (toluene, sealed tube) which is hydrolyzed to alcohol 12 with ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
. This hydroxyl group has the wrong stereochemistry and is therefore inverted in a Mitsunobu reaction
Mitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate or diisopropyl azodicarboxylate . The alcohol undergoes an inversion of stereochemistry...
with p-nitrobenzoic acid followed by hydrolysis of the p-nitrobenzoate to 13.
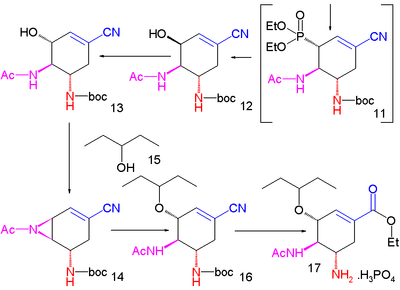
A second Mitsunobu reaction then forms the aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
14 available for ring-opening reaction with 3-pentanol
3-Pentanol
3-Pentanol is one of the isomers of amyl alcohol....
catalyzed by boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
to ether 15. In the final step the BOC group is removed (HCl) and phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
added to objective 16.
Fukuyama synthesis
An approach published in 2007 like Corey's starts by an asymmetric Diels-Alder reaction this time with starting materials pyridinePyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
and acrolein
Acrolein
Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity...
.
 |
 |
|
| Fukuyama Tamiflu synthesis Part I | Part II | |
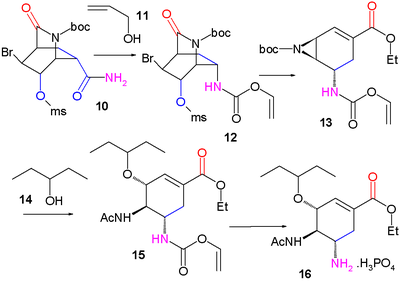
Pyridine (1) is reduced with sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
in presence of benzyl chloroformate
Benzyl chloroformate
Benzyl chloroformate is the benzyl ester of chloroformic acid. It is also known as benzyl chlorocarbonate is an oily liquid whose color is anywhere from yellow to colorless. It is also known for its pungent odor...
to the Cbz
Carboxybenzyl
Carboxybenzyl or Cbz or Z is an amine protecting group in organic synthesis. It is commonly used in peptide synthesis and is formed by reacting an amine with benzyl chloroformate and a weak base:It is used to protect amines from electrophiles...
protected dihydropyridine 2. The asymmetric Diels-Alder reaction with acrolein
Acrolein
Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity...
3 is carried out with the McMillan catalyst to the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
4 as the endo isomer which is oxidized to the carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
5 with sodium chlorite
Sodium chlorite
Sodium chlorite is a chemical compound used in the manufacture of paper.-Use:The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and paper. It is also used for disinfection of a few municipal water treatment plants after...
, Monopotassium phosphate
Monopotassium phosphate
Monopotassium phosphate -- 24 -- is a soluble salt which is used as a fertilizer, a food additive and a fungicide. It is a source of phosphorus and potassium. It is also a buffering agent...
and 2-methyl-2-butene. Addition of bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
gives halolactonization product 6 and after replacement of the Cbz protective group by a BOC
BOC
BOC may refer toBanks:* Bank of Canada, Canada's central bank* Bank of China, a major state-owned bank in the People's Republic of China* Bank of Ceylon, a major government-owned commercial bank* Bank of Cyprus, a major cypriot financial institution...
protective group in 7 (hydrogenolysis
Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
in the presence of Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. This carbonate ester reacts with amines to give N-tert-butoxycarbonyl or so-called t-BOC derivatives. These derivatives do not behave as amines, which allows certain subsequent transformations to occur that would have...
) a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group is introduced in intermediate 8 by catalytic ruthenium(IV) oxide
Ruthenium(IV) oxide
Ruthenium oxide is a black chemical compound containing the rare metal ruthenium and oxygen. The most often used O2 catalyst is ruthenium oxide; however, care must be taken since hydrates of this oxide exist....
and sacrificial catalyst sodium periodate
Sodium periodate
Sodium periodate is the sodium salt of periodic acid. It can refer to two different chemical compounds, sodium metaperiodate , which has the formula NaIO4, and sodium orthoperiodate , which has the formula Na2H3IO6...
. Addition of ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
cleaves the ester group to form amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
9 the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
group of which is mesylated to compound 10. In the next
step iodobenzene diacetate is added, converting the amide in a Hofmann rearrangement
Hofmann rearrangement
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atom.The reaction is named after its discoverer: August Wilhelm von Hofmann...
to the allyl carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
12 after capturing the intermediate isocyanate with allyl alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
11. On addition of sodium ethoxide
Sodium ethoxide
Sodium ethoxide is an alkoxide salt with the chemical formula C2H5ONa.-Preparation:It is commercially available as a white solid, or as a solution in ethanol. It is easily prepared in the laboratory by reacting sodium metal with ethanol:...
in ethanol three reactions take place simultaneously: cleavage of the amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
to form new an ethyl ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
group, displacement of the mesyl group by newly formed BOC protected amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
to an aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
group and an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
forming the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
group in 13 with liberation of HBr. In the final two steps the aziridine ring is opened by 3-pentanol
3-Pentanol
3-Pentanol is one of the isomers of amyl alcohol....
14 and boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
to aminoether 15 with the BOC group replaced by an acyl
Acyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
group and on removal of the other amine protecting group (Pd/C
Palladium on carbon
Palladium on carbon, often referred to as Pd/C, is a form of palladium used for catalysis. It is usually used for catalytic hydrogenations in organic chemistry...
, Ph3P
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
, and 1,3-dimethylbarbituric acid
Barbituric acid
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active...
in ethanol) and addition of phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
oseltamivir 16 is obtained.
Trost synthesis
In 2008 the group of Barry M. Trost of the Stanford University published the shortest synthetic route to date .