Organoselenium chemistry
Encyclopedia
Organoselenium compounds are chemical compound
s containing carbon
-to-selenium
chemical bond
s. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements and similarities in chemistry are to be expected.
Selenium can exist with oxidation state
-2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength
becomes increasingly weaker (234 kJ/mol
for the C–Se bond and 272 kJ/mol for the C–S bond) and the bond length
s longer (C–Se 198 pm, C–S 181 pm and C–O 141 pm). Selenium compounds are more nucleophilic than the corresponding sulfur compounds and also more acidic. The pKa
values of XH2 are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxide
s, the corresponding selenoxides are unstable in the presence of β-protons and this property is utilized in many organic reaction
s of selenium, notably in selenoxide oxidations and in selenoxide eliminations. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
The first organoselenium compound ever isolated was diethylselenide in 1836.
is a selenol-containing amino acid that is encoded in a special manner by DNA. Selenomethionine
is a selenide-containing amino acid that also occurs naturally, but is generated by post-transcriptional modification. Glutathione oxidase
is an enzyme with a diselenide at its active site.
of selenophenol to alkynes affords, preferentially, the Z-vinylic selenides after longer reaction times at room temperature.The reaction is faster at a high temperature; however, the mixture of Z- and E-vinylic selenides was obtained in an almost 1:1 ratio. On the other hand, the adducts depend on the nature of the substituent
s at the triple bond
. Conversely, vinylic selenides can be prepared by palladium
-catalyzed hydroselenation of alkynes to afford the Markownikov adduct in good yields. There are some limitations associated with the methodologies to prepare vinylic selenides illustrated above; the procedures described employ diorganoyl diselenides or selenophenol as starting materials, which are volatile and unstable and have an unpleasant odor. Also, the preparation of these compounds is complex.
is useful in organic oxidation. Specifically, SeO2 will convert an allylic methylene
group into the corresponding alcohol
. A number of other reagents bring about this reaction.
.
In terms of reaction mechanism
, SeO2 and the allylic substrate react via pericyclic process beginning with an ene reaction
that activates the C-H bond. The second step is a [2,3] sigmatropic reaction
. Oxidations involving selenium dioxide are often carried out with catalytic amounts of the selenium compound and in presence of a sacrificial catalyst or co-oxidant such as hydrogen peroxide
.
SeO2-based oxidations sometimes afford carbonyl compounds such as ketone
s., β-Pinene
and cyclohexanone
oxidation to 1,2-cyclohexanedione Oxidation of ketone
s having α-methylene groups affords diketones. This type of oxidation with selenium oxide is called Riley oxidation.
after oxidation, to leave behind an alkene
and a selenol. In the elimination reaction, all five participating reaction centers are coplanar and, therefore, the reaction stereochemistry is syn. Oxidizing agents used are hydrogen peroxide
, ozone
or MCPBA. This reaction type is often used with ketone
s leading to enone
s. An example is acetylcyclohexanone elimination with benzeneselenylchloride and sodium hydride
 The Grieco elimination
The Grieco elimination
is a similar selenoxide elimination using o-nitrophenylselenocyanate and tributylphosphine to cause the elimination of the elements of H2O.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s containing carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-to-selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements and similarities in chemistry are to be expected.
Selenium can exist with oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
-2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength
Bond strength
In chemistry, bond strength is measured between two atoms joined in a chemical bond. It is the degree to which each atom linked to another atom contributes to the valency of this other atom...
becomes increasingly weaker (234 kJ/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
for the C–Se bond and 272 kJ/mol for the C–S bond) and the bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s longer (C–Se 198 pm, C–S 181 pm and C–O 141 pm). Selenium compounds are more nucleophilic than the corresponding sulfur compounds and also more acidic. The pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
values of XH2 are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
s, the corresponding selenoxides are unstable in the presence of β-protons and this property is utilized in many organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s of selenium, notably in selenoxide oxidations and in selenoxide eliminations. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
The first organoselenium compound ever isolated was diethylselenide in 1836.
Structural classification of organoselenium compounds
- SelenolSelenolSelenols are organic compounds that contain the functional group with the connectivity C-Se-H. Selenols are sometimes also called selenamercaptans, selenathiols, and selenothiols. Selenols are one of the principal classes of organoselenium compounds...
s (RSeH) are the selenium equivalents of alcoholAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s and thiolThiolIn organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
s. These compounds are relatively unstable and generally have an unpleasant smell. PhenylselenolBenzeneselenolBenzeneselenol is the chemical compound with the formula C6H5SeH, often abbreviated PhSeH. This intensely malodorous liquid is a useful reagent in organic synthesis.-Synthesis and basic properties:...
(also called selenaphenol or PhSeH) is more acidic (pKa 5.9) than thiophenol (pKa 6.5) and also oxidizes more readily to the diselenide. Selenaphenol is prepared by reduction of diphenyldiselenide. - Diselenides (R-Se-Se-R) are the selenium equivalents of peroxidePeroxideA peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
s and disulfideDisulfideIn chemistry, a disulfide usually refers to the structural unit composed of a linked pair of sulfur atoms. Disulfide usually refer to a chemical compound that contains a disulfide bond, such as diphenyl disulfide, C6H5S-SC6H5....
s. They are useful shelf-stable precursors to more reactive organoselenium reagents such as selenols and selenenyl halides. Best known in organic chemistry is diphenyldiselenide, prepared from phenylmagnesium bromidePhenylmagnesium bromidePhenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is so commonly used that it is commercially available as a solution in diethyl ether or tetrahydrofuran . Phenylmagnesium bromide is a Grignard reagent...
and selenium followed by oxidation of the product PhSeMgBr. - Selenenyl halides (R-Se-Cl, R-Se-Br) are prepared by halogenation of diselenides. Bromination of diphenyldiselenide gives phenylselenyl bromide (PhSeBr). These compounds are sources of "PhSe+".
- Selenides (R-Se-R), also called selenoethers, are the selenium equivalents of etherEtherEthers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s and thioetherThioetherA thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
s. These are the most prevalent organoselenium compounds. Symmetrical selenides are usually prepared by alkylation of alkali metal selenide salts, e.g. sodium selenideSodium selenideSodium selenide is a inorganic compound of sodium and selenium with the chemical formula Na2Se. This colourless solid is prepared by the reaction of selenium with a solution of sodium in ammonia....
. Unsymmetrical selenides are prepared by alkylation of selenoates. These compounds are typically react as a nucleophileNucleophileA nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s, e.g. with alkyl halides (R'-X) to give selenonium salts R'RRSe+X-. Divalent selenium can also interact with soft heteroatoms to form hypervalent selenium centers. They also react in some circumstances as electrophiles, e.g. with organolithium reagents (R'Li) to the ate complexAte complexAn ate complex in chemistry is a salt formed by reaction of a Lewis acid with a base whereby the central atom increases its valence . Often in chemical nomenclature the phrase ate is suffixed to the element in question. For example, the ate complex of a boron compound is called a borate...
R'RRSe-Li+. - Selenoxides (R-Se(O)-R) are the selenium equivalents of sulfoxideSulfoxideA sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
s. They can be further oxidized to selenones R-Se(O)2R, the selenium analogues of sulfoneSulfoneA sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
s. - Perseleninic acids (RSe(O)OOH) catalyse epoxidation reactions and Baeyer–Villiger oxidations.
- Selenuranes are hypervalent organoselenium compounds, formally derived from the tetrahalides such as SeCl4. Examples are of the type ArSeCl3. The chlorides are obtained by chlorination of the selenenyl chlorideSelenium oxydichlorideSelenium oxydichloride is the inorganic compound with the formula SeOCl2. It is a liquid with a high dielectric constant and high specific conductance, and for these reasons is an attractive solvent...
. - Seleniranes are three-membered rings (parent: C2H4Se) related to thiiraneEpisulfideEpisulfides are a class of organic compounds that contain a saturated heterocyclic ring consisting of two carbon atoms and one sulfur atom. It is the sulfur analogue of an epoxide or aziridine. They are also known as thiiranes, olefin sulfides, thioalkylene oxides, and thiacyclopropanes.The parent...
s but, unlike thiiranes, seleniranes are kinetically unstable, extruding selenium directly (without oxidation) to form alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s. This property has been utilized in synthetic organic chemistry. - SeloneSeloneIn chemistry, a selone is the structural analog of a ketone where selenium replaces oxygen. Selones are used as chiral derivatizing agents for selenium-77 NMR spectroscopy. Chiral oxazolidineselones are excellent partners in the stereospecific aldol reaction, and 77Se NMR can verify the...
s (R2C=Se, sometimes called selenones) are the selenium analogues of ketones. They are rare because to their tendency to oligomerOligomerIn chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
ize. Diselenobenzoquinone is stable as a metal complex SelenoureaSelenoureaSelenourea is the organoselenium compound with the formula SeC2. It is a white solid. This compound features a rare example of a stable, unhindered carbon-selenium double bond. The compound is used in the synthesis of selenium heterocycles...
is an example of a stable compound containing a C=Se bond.
Organoselenium compounds in nature
Selenium is required for life, albeit only in small amounts. SelenocysteineSelenocysteine
Selenocysteine is an amino acid that is present in several enzymes .-Nomenclature:...
is a selenol-containing amino acid that is encoded in a special manner by DNA. Selenomethionine
Selenomethionine
Selenomethionine is an amino acid containing selenium. The L-enantiomer of selenomethionine, known as Se-met and Sem, is a common natural food source of selenium. In vivo, selenomethionine is randomly incorporated instead of methionine and is readily oxidized. Its antioxidant activity arises from...
is a selenide-containing amino acid that also occurs naturally, but is generated by post-transcriptional modification. Glutathione oxidase
Glutathione oxidase
In enzymology, a glutathione oxidase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are glutathione and O2, whereas its two products are glutathione disulfide and H2O2....
is an enzyme with a diselenide at its active site.
Organoselenium chemistry in organic synthesis
Organoselenium compounds are specialized but useful collection of reagents useful in organic synthesis, although they are generally excluded from processes useful to pharmaceuticals owing to regulatory issues. Their usefulness hinges on certain attributes, including (i) the weakness of the C-Se bond and (ii) the easy oxidation of divalent selenium compounds.Vinylic selenides
Vinylic selenides are organoselenium compounds that play a role in organic synthesis, especially in the development of convenient stereoselective routes to functionalized alkenes. Although various methods are mentioned for the preparation of vinylic selenides, a more useful procedure has centered on the nucleophilic or electrophilic organoselenium addition to terminal or internal alkynes. For example, the nucleophilic additionNucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of selenophenol to alkynes affords, preferentially, the Z-vinylic selenides after longer reaction times at room temperature.The reaction is faster at a high temperature; however, the mixture of Z- and E-vinylic selenides was obtained in an almost 1:1 ratio. On the other hand, the adducts depend on the nature of the substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s at the triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
. Conversely, vinylic selenides can be prepared by palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
-catalyzed hydroselenation of alkynes to afford the Markownikov adduct in good yields. There are some limitations associated with the methodologies to prepare vinylic selenides illustrated above; the procedures described employ diorganoyl diselenides or selenophenol as starting materials, which are volatile and unstable and have an unpleasant odor. Also, the preparation of these compounds is complex.
Selenoxide oxidations
Selenium dioxideSelenium dioxide
Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.-Properties:...
is useful in organic oxidation. Specifically, SeO2 will convert an allylic methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
group into the corresponding alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
. A number of other reagents bring about this reaction.
.
In terms of reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
, SeO2 and the allylic substrate react via pericyclic process beginning with an ene reaction
Ene reaction
The Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the...
that activates the C-H bond. The second step is a [2,3] sigmatropic reaction
Sigmatropic reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular process. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon-carbon...
. Oxidations involving selenium dioxide are often carried out with catalytic amounts of the selenium compound and in presence of a sacrificial catalyst or co-oxidant such as hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
.
SeO2-based oxidations sometimes afford carbonyl compounds such as ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s., β-Pinene
Pinene
Pinene is a bicyclic monoterpene chemical compound. There are two structural isomers of pinene found in nature: α-pinene and β-pinene. As the name suggests, both forms are important constituents of pine resin; they are also found in the resins of many other conifers, as well as in non-coniferous...
and cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
oxidation to 1,2-cyclohexanedione Oxidation of ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s having α-methylene groups affords diketones. This type of oxidation with selenium oxide is called Riley oxidation.
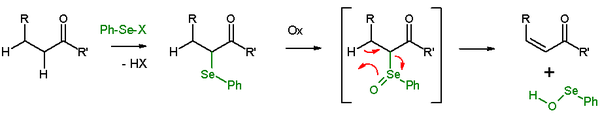
Selenoxide eliminations
In presence of a β-proton, a selenide will give an elimination reactionElimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
after oxidation, to leave behind an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and a selenol. In the elimination reaction, all five participating reaction centers are coplanar and, therefore, the reaction stereochemistry is syn. Oxidizing agents used are hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
or MCPBA. This reaction type is often used with ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s leading to enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
s. An example is acetylcyclohexanone elimination with benzeneselenylchloride and sodium hydride
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...

Grieco elimination
The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene ....
is a similar selenoxide elimination using o-nitrophenylselenocyanate and tributylphosphine to cause the elimination of the elements of H2O.
See also
- The chemistry of carbon bonded to other elements in the periodic table:

