Organonickel
Encyclopedia

Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
that deals with organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s feature nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly put to use in the Mond process
Mond process
The Mond process, sometimes known as the carbonyl process is a technique created by Ludwig Mond in 1890 to extract and purify nickel. The process was used commercially before the end of the 19th century...
for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
s, hydrocyanation
Hydrocyanation
Hydrocyanation is, most fundamentally, the process whereby H+ and –CN ions are added to a molecular substrate. Usually the substrate is an alkene and the product is a nitrile. When –CN is a ligand in a transition metal complex, its basicity makes it difficult to dislodge, so, in this...
, and the Shell higher olefin process
Shell higher olefin process
The Shell higher olefin process is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell. The olefin products are converted to fatty aldehydes and then to fatty alcohols, which are precursors...
.
Overview
Organonickel compounds adopts oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
0 or +2. The resemblance to organopalladium
Organopalladium
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond...
compounds is not strong, although both metals undergoe reactions that involve sequences of reductive elimination and oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
reactions.
Ni alkene complexes
Many complexes exist of nickel coordinated to an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. In these compounds nickel is formally zerovalent Ni0 and the bonding is described with the Dewar-Chatt-Duncanson model
Dewar-Chatt-Duncanson model
The Dewar–Chatt–Duncanson model is a model in organometallic chemistry which explains the type of chemical bonding between an alkene and a metal in certain organometallic compounds. The model is named after Michael J. S. Dewar, Joseph Chatt and L. A...
. One common representative is Bis(cyclooctadiene)nickel(0)
Bis(cyclooctadiene)nickel(0)
Bisnickel is the organometallic compound with the formula Ni2. This air-sensitive yellow solid is a common source of Ni in chemical synthesis....
(Ni(COD)2), which contains two cyclooctadiene
Cyclooctadiene
A cyclooctadiene is a cyclic diene with the formula C8H12. Focusing only on cis derivatives, four isomers are possible: 1,2, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers are 1,3-cyclooctadiene and 1,5-cyclooctadiene, which is used as a ligand for transition...
ligands. It is a 18VE compound with 10 electrons provided by nickel itself and 4x2 electrons more by the double bonds. This solid, which melts at 60 °C, is used as a catalyst.
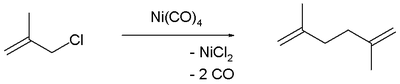
Ni allyl complexes
nickel(ii).png)
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
halides react with Ni(CO)4 to form pi-allyl complexes, (allyl)2Ni2Cl2. These compounds in turn are sources of allyl nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s. In (allyl)2Ni2Cl2 and (allyl)Ni(C5H5), nickel is assigned to oxidation number
Oxidation number
In coordination chemistry, the oxidation number of a central atom in a coordination compound is the charge that it would have if all the ligands were removed along with the electron pairs that were shared with the central atom. Oxidation numbers are often confused with oxidation states.The...
+2, and the electron counts are 16 and 18, respectively.
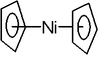
Nickelocene
Nickelocene
Nickelocene
Nickelocene is the organonickel compound with the formula Ni2. Also known as bisnickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it yet has no practical applications....
NiCp2 with +2 Ni oxidation state and 20 valence electrons is the main metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
of nickel. It can be oxidized by one electron. The corresponding palladocene and platinocene are unknown.
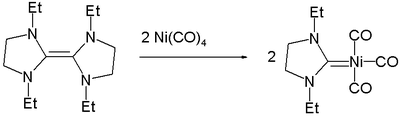
Nickel carbene complexes
Nickel forms carbeneTransition metal carbene complex
A transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and...
complxes, formally featuring C=Ni double bonds.
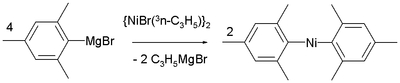
Nickel 12 VE compounds
Nickel compounds of the type NiR2 also exist with just 12 valence electrons. In solution however solvent molecules always interact with the metal atom increasing the electron count. One true 12 VE compound is di(mesityl)nickel prepared from (allyl)2Ni2Br2 and the corresponding Grignard reagent.Alkene/alkyne oligomerizations
Nickel compounds catalyze the oligomerization of alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s and alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s. This property came to light as part as the development of Ziegler-Natta catalyst
Ziegler-Natta catalyst
A Ziegler–Natta catalyst is a catalyst used in the synthesis of polymers of 1-alkenes . Three types of Ziegler–Natta catalysts are currently employed:* Solid and supported catalysts based on titanium compounds...
in the 1950s. It was found that nickel impurities originating from an autoclave
Autoclave
An autoclave is an instrument used to sterilize equipment and supplies by subjecting them to high pressure saturated steam at 121 °C for around 15–20 minutes depending on the size of the load and the contents. It was invented by Charles Chamberland in 1879, although a precursor known as the...
killed the propagation reaction (Aufbau) in favor of termination reaction to a terminal alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
: the polymerization of ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
simply stopped at 1-butene
1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
. This so-called nickel effect prompted the search for other catalyst capable of this reaction which resulted in the discovery of new catalysts that actually gave high molar mass polymers (the actual Ziegler-Natta catalysts).
One practical implementation of alkyne oligomerization is the Reppe synthesis for example in the synthesis of cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
:
This is a formal [2+2+2+2]cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
. The oligomerization of butadiene with ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
to trans-1,4-hexadiene at one time was an industrial process.
Formal [2+2+2] cycloadditions also take place in alkyne trimerisation
Alkyne trimerisation
An alkyne trimerisation reaction is a 2+2+2 cyclization reaction in which three alkyne molecules react to form an aromatic compound. The reaction is 'pseudo' pericyclic since it has not been observed to occur without the assistance of metal catalysis; and the metal catalyst assembles the ring...
. This trimerisation can be extended to inclusion of benzyne. Benzyne is generated in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
from a benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
compound with a triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
and a trimethylsilyl
Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
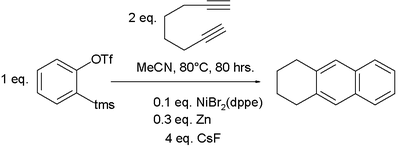
in the ortho- positions and reacts with a di-yne such as 1,7-octadiyne and with a nickel(II) bromide
Nickel(II) bromide
Nickel bromide, NiBr2, is the nickel salt of hydrobromic acid. It can be made by reacting nickel, nickel oxide, nickel carbonate, or nickel hydroxide with hydrobromic acid. It can also be made by reacting nickel with bromine. It is a weak reducing agent.It is yellow-brown, rhombohedral,...
/ zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
catalyst system (NiBr2 bis(diphenylphosphino) ethane
1,2-Bis(diphenylphosphino)ethane
1,2-Bisethane is a commonly used bidentate ligand in coordination chemistry. Dppe is almost invariably chelated, although there are examples of unidentate and of bridging behavior.-Preparation:...
/ Zn) to the corresponding naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
derivative.
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
elementary zinc serves to reduce nickel(II) to nickel(0) to which can then coordinate two alkyne bonds. A cyclometalation step follows to the nickelcyclopentadiene intermediate and then coordination of the benzyne which gives a C-H insertion reaction to the nickelcycloheptatriene compound. Reductive elimination liberates the tetrahydroanthracene compound.
The formation of organonickel compounds in this type of reaction is not always obvious but in a carefully designed experiment two such intermediates are formed quantitatively :
It is noted in one study that this reaction only works with acetylene itself or with simple alkynes due to poor regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
. From a terminal alkyne 7 isomers are possibly differing in the position of the substituents or the double bond positions. One strategy to remedy this problem employs certain diynes:
The selected reaction conditions also minimize the amount formed of competing 2+2+2cycloaddition product to the corresponding substituted arene.
Coupling reactions
Nickel compounds cause the coupling reactionCoupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
between allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
and aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s. Other coupling reactions involving nickel in catalytic amounts
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
are the Kumada coupling
Kumada coupling
A Kumada coupling or Kumada-Corriu coupling is a cross coupling reaction in organic chemistry between an alkyl or aryl Grignard reagent and an aryl or vinyl halocarbon catalysed by nickel or palladium. This reaction is relevant to organic synthesis because it gives access to styrene derivatives...
and the Negishi coupling
Negishi coupling
The Negishi coupling is a cross coupling reaction in organic chemistry involving an organozinc compound, an organic halide and a nickel or palladium catalyst creating a new carbon-carbon covalent bond:* The halide X can be chloride, bromine or iodine but also a triflate or acetyloxy group with as...
.
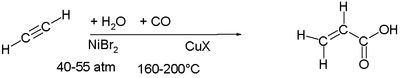
Ni carbonylation
Ni catalyzes the addition of carbon monoxideCarbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
to alkenes and alkynes. The industrial production of acrylic acid
Acrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
at one time consisted of combining acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
, carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
at 40-55 atm and 160-200°C with nickel(II) bromide
Nickel(II) bromide
Nickel bromide, NiBr2, is the nickel salt of hydrobromic acid. It can be made by reacting nickel, nickel oxide, nickel carbonate, or nickel hydroxide with hydrobromic acid. It can also be made by reacting nickel with bromine. It is a weak reducing agent.It is yellow-brown, rhombohedral,...
and a copper halide.
See also
- Compounds of carbon with other elements in the periodic table:


nickel(0).png)